Phase equilibria of pie crust
With American Thanksgiving and Christmas rapidly approaching, the pie baking season is rapidly approaching. One of the most important, but least quantified, aspects of pie creation is the crustal composition. A simple ternary phase diagram for three-phase pie crust is presented below.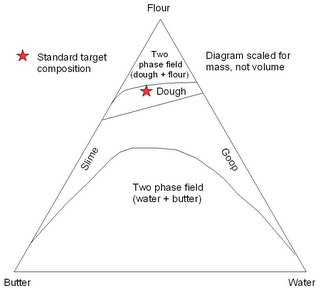
While the “traditional composition” point is plotted to scale, the positions and shapes of the curve are poorly constrained approximations. Lack of accurate thermodynamic data for the system precludes accurate prediction of these fields. It is the shapes and positions of the top two curves that is of paramount importance; anyone who reaches the butter-water two phase field should be banished from the kitchen.
As anyone with baking experience knows, the stability region for pie crust is a relatively small area on the wet side of the two phase flour + dough field. This field is generally approached by adding water to a flour/butter mixture, as is shown below.
However, if the approximated slopes shown above are correct, then a radical new approach to crustal formation might be advisable. By generating a flour-water mixture, and then adding butter, a wider range of valid crustal compositions should be achievable before exiting the edible portion of this phase diagram. This approach is shown below.
I will be baking at least three pies this week for T-day. I might have a go at this radical new approach, to see if it yields additional information on the slope of the upper two phase boundaries. I suspect they might be steeper than the lines shown here. Assuming I can actually get a cheap, accurate kitchen scale, there may be more reports on the thermopienamics of this system sometime next week.
9 comments:
Haha! Fantastic. I may have to print out the diagrams and put them on the kitchen wall.
Brilliant. I wonder how many other culinary systems are amenable to a ternary analysis? Replace water with milk and we can investigate the stability field for bechemal sauce...
Cake, on the other hand, is a four phase system.
This is why I use graham cracker crusts - there's a much greater range of stable compositions. Also, it means one of my endmembers is sugar.
Can you do lids with a graham cracker crust, or does that component force you to work in an open-face system?
If by "lid" you mean "meringue", and I don't see why you shouldn't, then you can.
If you get the chance, take a small portion of the mixture and push through the flour-poor dough phase boundary into the miscible slime-goop field and then down into the butter-water two phase field. I notice you have plotted a boundary with a symmetrical shape, and it has gotten me thinking about the partitioning behaviour of flour between immiscible butter and water. Will flour partition equally between the two? Is flour butyrophile? I have no feeling for how flour should behave. Clearly, experimental investigation is required.
Anonymous: we discourage playing with food in our house, and anything in the lower 2 phase field is far enough away from the edibility field to be considered playing.
Yami: I'm only a fan of meringues in certain traditional settings, like lemon or variations on the citrus custard. Apple pie with meringue instead of a crustal lid is, pardon the phrase, unamerican.
I sent a friend of mine the link to this post. Part of his reply was:
While I concur with the fundamental validity of the researcher's
paradigm, this respondent must take issue with an egregious failure
of experimental design: the principal components of experimental
manipulation (referred to in shorthand as "butter" and "flour") lack
adequate specificity of definition.
Thus, for instance, secondary researchers attempting to achieve
confirmatory replication might find confounding factors in
variability of the "flour": related to protein content of the
pulverized grain product, the particle size of the product as
employed in the laboratory, hygrodensity (known to some by the
popular term "moistness"), and perhaps most crucially, species origin
of the specific pulverized product being employed.
The firm lipid in the study, "butter", is also inadequately
specified. In classic expositions by earlier researchers, (see, for
examples, Rombauer, Rombauer, and Becker, various editions; Child, J;
or Beard, J), crucial distinctions are made between the cow-derived
substance as modified with sodium chloride, and that without;
substances of similar nature derived from fatty fluids derived from
other mammals; and even variations in cow-derived fats of diverse
geographical origin. Most crucially, however, many researchers, and
their critics, have posited an optimum response when the fatty
element in the compound is itself comprised of both a mammal-sourced
component and a component of semi-hardened vegetable-sourced fats,
referred to in some documents as "shortening". When employed without
admixture of this latter fat, "butter" alone can produce a
compositional character sometimes described as "tough". This term,
itself, also would benefit from further operational definition, but
that definition is beyond the scope of this paper, and is recommended
to other researchers.
Ooops.
Here is the text without excessive linebreaks:
While I concur with the fundamental validity of the researcher's paradigm, this respondent must take issue with an egregious failure of experimental design: the principal components of experimental manipulation (referred to in shorthand as "butter" and "flour") lack adequate specificity of definition.
Thus, for instance, secondary researchers attempting to achieve confirmatory replication might find confounding factors in variability of the "flour": related to protein content of the pulverized grain product, the particle size of the product as employed in the laboratory, hygrodensity (known to some by the popular term "moistness"), and perhaps most crucially, species origin of the specific pulverized product being employed.
The firm lipid in the study, "butter", is also inadequately specified. In classic expositions by earlier researchers, (see, for examples, Rombauer, Rombauer, and Becker, various editions; Child, J; or Beard, J), crucial distinctions are made between the cow-derived substance as modified with sodium chloride, and that without; substances of similar nature derived from fatty fluids derived from other mammals; and even variations in cow-derived fats of diverse geographical origin. Most crucially, however, many researchers, and their critics, have posited an optimum response when the fatty element in the compound is itself comprised of both a mammal-sourced component and a component of semi-hardened vegetable-sourced fats, referred to in some documents as "shortening". When employed without admixture of this latter fat, "butter" alone can produce a compositional character sometimes described as "tough". This term, itself, also would benefit from further operational definition, but that definition is beyond the scope of this paper, and is recommended to other researchers.
Post a Comment