Merry Christmas
The rest of the Lemmings and I hope that everyone on the internet has a wonderful Christmas and a happy New Year.
I'm a geochemist. My main interest is in-situ mass spectrometry, but I have a soft spot in my heart for thermodynamics, poetry, drillers, trees, bicycles, and cosmochemistry.
The rest of the Lemmings and I hope that everyone on the internet has a wonderful Christmas and a happy New Year.
Posted by
C W Magee
at
7:05 AM
0
comments
![]()
Labels: Just sayin'

when you catch yourself checking out the goannas.
p.s. The toenail polish is a nice touch.
p.p.s. I'm adding photos to previous posts which I didn't manage to upload at the time, so old posts might appear in the RSS feed. You've been warned.
Posted by
C W Magee
at
10:54 PM
0
comments
![]()
Labels: Outback Lemming
How'd you like to do a field project on this 1x1.5 degree mapsheet?
Posted by
Dr. Lemming
at
10:18 AM
5
comments
![]()
Labels: Outback Lemming

My first trophy rock is a 4 kg boulder of the Lavras conglomerate from the Tombador formation of the mid-Proterozoic Espinhaço Supergroup. This is a well-rounded, poorly sorted conglomerate with a clast size of 1-9 cm in this hand sample. The clasts are dominated by a pink arkose, with white and green arenites also present. The rock is clast- supported, and some clasts show evidence of minor pressure dissolution along their contacts. The matrix is slightly ferruginized. This rock is the purported source rock for the diamonds for the Lencois diamonds workings in Bahia state, Brazil.
My second trophy rock is a migmatite from the Wyoming craton. The strongly folded mesosome consists mostly of biotite and amphibole, with a minor opaque oxide phase. The leucosome follows the folds of the rock, but has equant, undeformed coarse-grained K-spar and quartz crystals, suggesting that crystallization was post-deformational.
The third trophy rock, part A, is a 45cm Cambrian stromatolite colony from an undisclosed location in Australia’s Georgoina basin. Part B is a smaller fragment of a contemporaneous stromatolite colony, with which has been progressively silicified, showing laminar color variations caused by trace elements in the chalcedony.
Unlike Brian’s rocks, these specimens are widely separated in space and time.
Posted by
C W Magee
at
11:20 AM
6
comments
![]()
In a dramatic bid to combat global warming, Santa Claus has announced that he will no longer use coal as a stocking stuffer for naughty children.
“As an Arctic resident, I am especially vulnerable to anthropogenic climate change,” said St. Claus at a press conference held at his North Pole workshop. “It is important that I lead by example in combating this problem.”
Santa’s elves considered several adaptive strategies to address global warming, including relocation to East Antarctica, and replacement of the reindeer with antelope, or manatees. But ultimately, they decided that a dramatic, proactive prevention effort was most in the spirit of Christmas. However, in a move certain to provoke controversy, the jolly fat man will be replacing the coal formerly given to naughty children with uranium oxide, the fuel for nuclear reactors.
Claus defends this decision, claiming that in addition to the reduced carbon footprint, the nuclear solution has two added benefits. Firstly, the higher energy density of uranium means that each recalcitrant child will only be receiving a thimbleful of fuel, instead of a large lump of coal- a change that will dramatically reduce the load on the sled. Secondly, Claus believes that the threat of receiving a radioactive stocking gift will act as a greater deterrent than coal ever did. “We looked into the renewable options,” said Claus, “but we feared that little solar cells and windmills might be mistaken for toys.”
The environmental movement’s response to this development has been mixed. Mainstream groups have described the change as “brave”, or “unfortunate but understandable”. Extremist fringe elements have been less equivocal.
“He will not be allowed in our airspace,” said the New Zealand defense minister, “unless he can certify that his sled is free of nuclear fuel.” When pressed about the prospect of denying his nation’s entire juvenile population of their Christmas presents, the minister merely muttered something about overpriced geese, impoverished young cripples, and Humbugs.
The Earth Liberation Front was more direct in its criticism. “Santa Claus embodies the materialist industrial culture that has brought our biosphere to the brink of destruction,” said their spokesman. Further probing revealed that their members had no plans to hang stockings anyway.
No matter how vituperative the reaction is, Santa says that yellowcake is here to stay. “We simply cannot afford to continue our support for the coal industry that threatens to melt our homes and workshops in a matter of decades”, says Claus. But he then added that “All the boys and girls can avoid getting nuclear fuel in their stockings this Christmas simply by being nice instead of naughty.”
Posted by
Dr. Lemming
at
11:37 AM
10
comments
![]()
Labels: Greenhouse goofiness, Superficial Shilling
I went to the post office during my holiday last week, to send myself some college books that are once again useful to me as a result of my recent job change. To my dismay, I found that the USPS no longer offers any surface mail services overseas. So the book rate no longer exists. Does anyone out there know of any alternative ways of shipping large volumes of books overseas affordably?
Posted by
C W Magee
at
7:49 PM
5
comments
![]()
Labels: Tricks for young players
According to today's New York Times, this year's Siemens science contest was won by girls in both the individual and team categories. Janelle Schlossberger and Amanda Marinoff, 17 year olds from Long Island, split the first prize in the team division, while Isha Himani Jain, 16, from Bethlehem, Pa. placed first in the individual category. The article notes that 11 of the 20 finalists were women, and that most finalists were from public schools, and had at least one parent who was a scientist.
Posted by
C W Magee
at
10:31 AM
0
comments
![]()
Labels: Just sayin'
There are a number of ways to lose one's US citizenship. These include:
-Taking up arms against the United States.
-Conviction of Treason.
-Purchasing an automobile made in France.
Mrs. Lemming likes the Peugeot's alphabet soup of safety features, while I like the fact that the fuel efficiency of this family car is similar to my first motorbike.
I took LLLL into Sydney on a cool 49 mpg the other day. Even with diesel, that ain't bad.
Posted by
C W Magee
at
9:25 AM
7
comments
![]()
Labels: Climactic considerations
![]() Serpentinites, rocks made mostly of serpentine, are formed when water reacts with the earth’s mantle at moderate to low temperatures. Serpentinites are not rare, but they have a number of unusual geochemical features. These include:
Serpentinites, rocks made mostly of serpentine, are formed when water reacts with the earth’s mantle at moderate to low temperatures. Serpentinites are not rare, but they have a number of unusual geochemical features. These include:
Posted by
Dr. Lemming
at
1:44 AM
0
comments
![]()

For an analyst, measuring things is easy. It is the stuff that we can’t measure that keeps us up at night. For example, if we measure something- correctly- then we know how much is there. But if we don’t measure something, what does that tell us? That it isn’t there? Or that our analytical technique is not capable of detecting it?
One invention that we use to address this problem is a construct called the detection limit. This is a statistical number that represents the amount of something that we think we should be able to detect, if it is there. Thus, if we don’t detect something, we don’t say that it isn’t there, we say that it is Below Detection Limit (or BDL). In electron microprobe analyses, this limit is generally taken to be 3 times the standard error of the background*, and the same definition has lazily been co-opted into laser ICP-MS analysis. So if you want to improve your detection limit, reducing the level or the noise of your background is a good place to start.
Of course, there are some hypothesizers who sometimes want an analyst to demonstrate the absence of something. This can be frustrating, as it is statistically impossible to measure something that isn’t there. All we can do is place lower limits on something.
A well formulated hypothesis will recognize this, and ask us instead to show that something is below a specific level. But every now and then, you encounter a coward or handwaver who tries to hide beneath the detection limit, by claiming, “Well, this doesn’t support my pet theory, but it isn’t completely ruled out because my holy grail element could simply be less abundant than I predicted or than you can measure.”
If their original hypothesis was that something would be at concentration X, and the analyst failed to achieve a detection limit of X, then that is indeed the analyst’s fault. But if the theoretician arbitrarily fudges his expected concentration downwards to lie beneath any detection limit thrown his way, that ain’t cool. Unless, of course, he is prepared to put the effort in to improve the analytical technique so that it obtains the detection limit he needs.
* I should know the reference to the paper that established this- a seminal EPMA paper from the late 60’s, I believe, but I can’t for the life of me remember the reference. Anyone?
Posted by
C W Magee
at
10:59 PM
0
comments
![]()
Just look at what Microsoft Word started thinking when I tried telling it about a test commonly used by oil people...
Posted by
C W Magee
at
1:23 AM
4
comments
![]()
Labels: Underconstrained extrapolations
A few months ago, I ditched my university job to become a project geologist for a small exploration company. One thing I’ve noticed from my travels is that many people don’t have a real good idea what industrial geologists do. I’m fairly new at this gig, so here’s the best description that I can think of at 1 in the morning.
Basically, I get paid to look for buried treasure. Unlike colonial Spanish contraband or grandma’s Easter eggs, the treasure we look for is natural, having formed through geological processes at some point in Earth’s history.
We use geochemistry to understand the processes that allow the mineral deposits to form, and then we use geophysics to try to detect them from the surface. Once we formulate a hypothesis for the location of an economically valuable concentration of our target mineral(s), we drill holes in the ground to test that hypothesis.
From a scientific perspective, I like the work because it is very big picture, drawing on all sorts of skillz and knowledge that I didn’t use as a lab technician. From a personal perspective, I enjoy the wide open spaces, the camaraderie of a small company, and the feeling of accomplishment that comes with a day of hard physical and intellectual work. The pay isn’t bad either.
Posted by
C W Magee
at
1:18 AM
4
comments
![]()
Labels: Daily grind (or polish)
Imagine a subtly different alternate universe. A universe where academic jobs were still cutthroat, but a universe where scientists spoke honestly about their ambitions, where scientific competition was as engrained in popular culture as romantic rivalry, and where Avril Lavigne is a promising young analyst:
Posted by
C W Magee
at
12:09 AM
11
comments
![]()
When the IPCC tries to calculate the number of people likely to lose their lives as a result of climate change, do they include this sort of fatality?
Posted by
C W Magee
at
11:14 PM
2
comments
![]()
Labels: Greenhouse goofiness, Irreproducible idiocy, Natural and synthetic disasters
As you can see from this website, the first rain since June fell in the central NT last week. 8 mm may not seem like much, but it means that the dirt roads will be turning to mud. Our field season will be suspended until the wet tails off in March or April. So I'll be sleeping under a roof until then.
Posted by
C W Magee
at
10:54 PM
0
comments
![]()
Labels: Outback Lemming
I bumped into climatecrisis.net's climate calculator via skookumchick's blog, but it appears to be a front for generic environmentalism, rather than a good-faith attempt to measure one's carbon emissions. Here is why:
It asks the question:
"What % of your electricity comes from clean, renewable sources such as solar, or wind?"
This question is only marginally relevant to greenhouse gas emissions. A much more perninent question for emissions would be
"What % of your electricity comes from hydro or nuclear?"
Hydroelectric power contributes about 19% of the world's electricity supply. Nuclear contributes about 11%. Solar and wind contribute less than 1% combined. So a person trying to accurately calculate the carbon emissions from their electricity use is much more likely to gain low carbon energy credit from hydro or nuclear than from wind or solar.
From the carbon cost point of view, all of these technologies generally have much lower emissions than fossil fuels, so there really isn't much of a good reason to distinguish between them for the purpose of calculating a climate footprint.
On the other hand, The environmental movement has long objected to hydro and nuclear for reasons that have nothing to do with GHG.
Therefore, one can only assume that the omission of 'dirty, non-renewable' low carbon energy sources from climatecrisis.net's list of low carbon energy sources is an indication that they are using climate change to push a broader environmental agenda at the expense of actual carbon emissions.
Posted by
C W Magee
at
9:04 PM
4
comments
![]()
See my previous post for the context of this discussion, and links to both papers.
There are two chief explanations for why the representation of women in senior scientific research roles is lower than would be statistically predicted.
The “Harvard hypothesis” suggests that women are intrinsically worse at doing science.
The “feminist hypothesis” suggests that women are discriminated against in one or more direct or indirect ways.
This comparison of recent noble gas diffusion papers provides data with which one can compare these competing hypotheses.
With regards to the Harvard hypothesis, Veronika’s paper is clearly better science than Bruce’s. Thus this hypothesis is contradicted, not supported, by comparing these two research reports.
Whether or not the feminist theory is supported or refuted is hard to tell. Clearly, Bruce got a poorer quality paper into a higher profile journal, but whether gender played a role in his ability to do so is unclear. Bruce is a senior, well-respected professor, while Veronica is a (relatively) early career researcher. So Bruce’s ability to get his paper into the higher profile journal could be a result of seniority, experience at the publication game, networking, or his previous track record. I will leave it to the reader to speculate whether gender impacts on any of these things, but I will say this: The editorial board of Nature does not seem to have neutralized any of these potentially biasing factors.
Posted by
Dr. Lemming
at
11:01 PM
2
comments
![]()
Labels: Scientific hoop-jumping
![]()
Thermochronic recently blogged about a high profile paper that recently suggested that the partitioning coefficients for argon between mantle minerals and melts is greater than one (Watson et al. 2007). As it turns out, Heber et al. 2007, published earlier this year, suggests that the D values are actually between 10-3 and 10-4 for all noble gasses. The Heber et al. 2007 paper describes a very well-thought out and executed research program, reads like a detective novel, and is generally everything that a scientific paper should be.
They perform a standard partitioning coefficient experiment- slowly cool a melt to grow crystals, quench it, and measure the concentration of the trace element of choice in both melt and crystal to determine the ratio. The brilliant thing about this study is the depth of self-critizism and examination which they perform to identify and eliminate potential contaminants or non-equilibrium effects. This stands in stark contrast to the Watson et al. paper, where they pretty much assume that their results are right, and arrive at a value 10,000 to 10 million times higher than the Heber et al. paper.
I don’t want to ruin a gripping read, but the things that Heber et al. overcome include (but are not limited to): Room temperature diffusion of He out of the glass between experiment completion and analysis time, bubble formation, melt inclusions, very low concentrations. The interpretations of the paper are well supported by the data, but do include an explanation of how these numbers can explain “primordial” OIB noble gas ratios.
Although the noble gas partition coefficients are low (on the order of 10-4), they are still 2 orders of magnitude higher than the partitioning coefficients of K, Th, and U, which are on the order of 10-6 in a hartzburgitic system. Thus, although noble gasses are incompatable, they are not as incompatable as the radioactive sources for 4He and 40Ar. So old depleted mantle will have a high primordial/radiogenic gas signature.
The fact that the Heber et al. and Watson et al. partition coefficients for argon differ by up to 8 orders of magnitude is striking. But I find the Heber et al. result more compelling, because the authors present, and then test, a dizzying array of potential complications in a rigorous manner. In contrast, Watson et al. make statements like,
“More generally, we believe that the inconsistency of our results and those of Broadhurst et al. with other experimental studies is due to the different experimental protocols used.”
While I have great respect for Professor Watson and his impressive research record, I am not interested in his beliefs, unless they are backed up by experimental evidence. And he makes no attempt to reconcile his results with previous work, much less try to disprove his own experiments. So a paper that dissects the potential causes of research discrepancies and addresses them is more convincing to me than one that relies on untested dismissals. As a final note, Watson et al. do not reference Heber et al. Heber et al. 2007 was published on 15 February, and available online since December 2006, while Watson et al. was not submitted until the beginning of May 2007.
References:
Veronika S. Heber, Richard A. Brooker, Simon P. Kelley, Bernard J. Wood. 2007 Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochimica et Cosmochimica Acta 71 1041-1061.
E. Bruce Watson, Jay B. Thomas, Daniele J. Cherniak 2007 40Ar retention in the terrestrial planets. Nature 449 299-304.
update: sociological speculations on these papers is here.
Posted by
Dr. Lemming
at
10:30 PM
9
comments
![]()
I have been learning all sorts of new and interesting geology in my new life as an exploration geologist, but one of the things I recently read surprised even me. Economic geology tends to be a conservative, observation-based branch of the discipline. Ideology and activism are very seldom seen. So you can imagine my astonishment when I found papers and reports where barium sulfate suddenly started to self-identify as “Baryte”. Needless to say, I was somewhat taken aback. BaSO4 is an insoluble, highly stable mineral, with a solidly orthorhombic space group of Pbmm. It is not one to subject itself to trendy postmodern spelling fads.
On the other hand, the alkali earths are on the far left fringes of the periodic table, second only to the erotic alkali. So perhaps the insidious nature of political correctness has corrupted their vacant electron shells. And old Europe’s IMA has evidently endorsed the feminist spelling, despite the fact that no self-respecting red blooded American mineralogist would consider typing it.
Of course, being French, they can’t even be consistent with themselves. Despite listing “Baryte” as the correct spelling, the IMA still officially calls the nitrate salt “Nitro-barite”. And nowhere do they use the name “Baryum” for element 56. So forget the fact that the ancient Greeks spelled it with a y thousands of years before they knew what an element was, this is a matter of principle. Just like sphene. I’m with webminerals.com and American Mineralogist on this one. So barite it is, and those left-wing euro-trash crystalogists should consider themselves lucky that I don’t call hydrated calcium sulfate “gipsum.”
p.s. Microsoft Word doesn’t recognize “baryte”, so I must be right.
Posted by
C W Magee
at
10:14 PM
2
comments
![]()
There are about a zillion periodic tables of the elements available in various formats and locations on and off the internet, but they never seem to actually have the info that one wants to see. Fortunately, Mr. Ash Norris, the electron probe technician at the Australian National University, has come up with a solution to this problem. He has a program that allows the user to print out a customized periodic table, containing whatever information one wishes to see. Check it out here.
Posted by
Dr. Lemming
at
9:09 PM
1 comments
![]()
Labels: Worth leaving the lounge
Here are five google search requests where this blog gets lucky:
sessile, omnivorous primates
thermodynamics of hot chicks
pseudoscience gender representation
hafnium is for lovers
Naked Mongols Always Slide Past Scantily Clad Argonauts
Full details here.
Posted by
C W Magee
at
10:07 PM
0
comments
![]()
Labels: Pompous proclamations
Thanks to a weather delay, I was still at Sydney airport when the Singapore Airlines A380 took off for the return flight of the maiden commercial flight of the very big plane. It was fun to watch, but the really cool thing was the people watching. 
Pilots coming off shift called home, saying they'd be late for dinner because they wanted to see the plane. All the guys on the tarmac who were supposed to be driving trucks or loading luggage all stopped, and positioned themselves for an ideal view. As for the plane itself, it was definitely bigger than a 747 (A british Air flight took off immediately afterwards), but it only looked really huge next to a 737 or other narrow body plane. It left the runway remarkably early for a plane of its size, but the climb angle was incredibly shallow- it took a very, very long time for it to disappear into the cloud deck.
There isn't a lot of old-fashioned, 20th century style bigger, faster, stronger engineering going on anymore these days, so it was cool to see a bit of it in action, in the form of the biggest passenger plane to enter service in 38 years.
Australia is supposed to get the first commercial dreamliner flights as well, but I doubt they will look as formidable. That's a 21st century plane.
I have pictures, but no way to post them at present...
Posted by
C W Magee
at
9:02 PM
0
comments
![]()
Labels: Just sayin'
Taking my fieldie to the airport this morning, we passed several cars racing in the World Solar Challenge. They were going slowly enough that they held up traffic- not surprising at 8:30 in the morning. Looking at the vehicles, we both thought the same thing- those cockpits must get HOT in the middle of the day. I doubt they have much in the way of air con. It turns out that the WSC has a blog. They might have pictures too- since I was behind the wheel, it wasn't practical for me to snap any.
Posted by
Dr. Lemming
at
4:56 PM
1 comments
![]()
Labels: Outback Lemming
So, I seem to have missed the boat on this volume of the geologic carnival, as it has been published and lithified over at Shear Sensibility. But I’ll post anyway, as a philosophical exercise.
There are two endmember philosophies used to look at geologic processes, particularly those related to geomorphology. One is catastrophism, the idea that sudden, discontinuous, dramatic events are the driving force in shaping Earth history. The opposite is uniformitarianism. This school of thought suggests that the processes we can observe happening regularly today can explain all the geological events worth investigating. The dramatic nature of the current accretionary theme lends itself to catastrophism, so I will try to shift the balance by describing a process that is common, widespread, and deadly.
Evaporation, and its biological buddy, transpiration, are the basis for the hydrologic cycle. Without evaporation, we would have no rain, snow, rivers, glaciers, or humidity, and the earth would be a cold, dry, and barren place to live. But too much of anything can kill, and transpiration is no exception.
The playas shown above are an instance where persistent evaporation can become unhealthy. Here, on the northern edge of the Simpson Desert, evaporation reigns supreme, and there is little or no available surface water for most of the year. In such a climate, a person will transpire readily. However, without a source of water to rehydrate, this transpiration can rapidly turn fatal.
There are two ways transpiration can kill. The first is heat stroke. As the body loses water, it becomes more difficult to maintain a constant body temperature in hot conditions. If the body starts to overheat, and there is no available water for evaporative cooling, death can come quickly, as the brain starts to sustain permanent damage at temperatures much over 40 C.
Even if temperatures aren’t so hot, dehydration caused by transpiration can still cause death. Loss of fluids thickens the blood, and puts strain on a number of bodily organs, particularly the kidneys. Left untreated, it can cause organ failure and death.
I’m off for the final field campaign of the season (I hope). Working in the middle of a stable craton with very subtle relief, it is unlikely that volcanoes, earthquakes, mass wasting, or other catastrophic events will kill me. But every summer people in Australia are killed by the toasty end of the hydrologic cycle. I’ll try to make sure my crew doesn’t suffer that fate.
Later, y’all.
Posted by
Dr. Lemming
at
11:37 PM
0
comments
![]()
Labels: Natural and synthetic disasters, Outback Lemming, Tricks for young players
Last field campaign, I was discussing my whale farm proposal with one of our fieldies. As a South Australian farmer, he’s naturally interesting in any sort of animal husbandry that doesn’t require rain, so after a few kilometers he pointed out an obvious inefficiency in my scheme.
In the original plan, mankind’s oil needs were to be supplied by the slaughter of 630 million sperm whales each year. This isn’t necessarily a problem- environmental groups are historically indifferent to the plight of marine mammals, but it does create a waste management issue.
Whales are only about 10-20% oil, so the mass killing of millions of them would rapidly produce an inconvenient pile of whale carcasses. If the blubber could be extracted from the whale without harming the animal, then this inefficiency can be solved.
Thus, whale liposuction is the obvious solution. Without having to worry about the herd’s growth rate, a steady state population can be maintained with a factor of 10 fewer animals. This the size of the planet to house them will be similarly reduced. It would still require 10 times more water than exists in Earth’s hydrosphere, but instead of having to use Ganymede as a whale breeding facility, we could get by with an icy body the size of Pluto, or Triton.
And there might be additional bonuses. The planet’s cadre of cosmetic veterinarians need never worry about unemployment again, and the prospect of seeing svelte, physically fit whales might broaden the pool of prospective whale watchers.
Posted by
Dr. Lemming
at
9:51 PM
3
comments
![]()
Labels: Greenhouse goofiness
One of the recurring themes in anti-global warming arguments is that earth scientists are hyping global warming so that they can get rich off of the increased research funding. The recent Nobel Prize results will probably initiate yet another round of motive questioning. Unlike some of the wishy-washier arguments made against climate change, this one is testable.
Suppose a college graduate in geology is looking to earn some money. Is climatology his best bet? For recent graduates, the next step towards a climatology career is a PhD. So, how do climatology PhD scholarships compare with other career options available to graduate geologists?
Well, PhD scholarships can be quite variable. This one gives A$19930 per year for three years. I found several other Australian universities that offer scholarships between $19,500 and $20,000 per year
Things in Europe are a bit more lucrative- this mob is offering 38,000 euros per year- more than twice as much.
I haven’t seen any American stipend figures in my brief net search, but my impression is that they are generally even lower than the Australian examples.
So, what other options does a freshly minted geologist have?
With a bachelor’s degree, a college grad is qualified for a junior position in the resource industry. But are these positions comparable with a PhD scholarship? After all, that European stipend looks pretty cushy. Here are a few entry level jobs advertised on seek.com.au, a popular Australian job site:
Coal mining: $100,000 - $160,000 (experience preferred, but graduates encouraged to apply)
Nickel- $85-$115K
Unknown type- $95-$125K (this actually asks for 1 year experience, so is not strictly a graduate level job)
Iron ore $80-$110K
Admittedly, most junior positions listed don’t name a figure, and people in the know say that $60,000-$75,000 is a more realistic expectation for a graduate with no work experience or advanced qualification. So there seems to be a selection bias in the ads that list potential salary figures. However, I expect that the same may be true of PhD programs.
So, if we assume a conservative Australian industry starting salary of $60,000, and compare that with an Australian PhD scholarship of $20,000, then it appears that those money grubbing climatologists are earning about a third of what they could get working in the resource industry.
Of course, climatologists won’t be students forever. In five years, they could be post-docs, earning about $50,000. In a comparable period of time, a senior geologist should be looking at about $150,000, still three times the academic salary.
Thus, the hypothesis that climatologists are in it for the money does not stand up to quantitative analysis. At least at the moment, an industrial career is several times more lucrative than an academic one.
Posted by
Dr. Lemming
at
1:11 AM
2
comments
![]()
Labels: Climactic considerations

Last break, I was sitting in a coffee shop, rocking LLLL’s stroller and sipping at a flat white. A woman with a not-quite-toddler came up to me and asked, “So, baby sitting today?”
No.
Babysitting is looking after somebody else’s child. Looking after one’s own child is called parenting. Or in my case, fathering. The number of people who refer to a dad looking after a baby as babysitting is actually a bit surprising. And annoying.
Babysitters get free pizza, pocket money, and access to movies that their folks don’t let them watch at home. It is a commercial transaction. Parents don’t get paid- we do it all for love. A dad doing his parental duty should not be such a rare sight that people assume he’s getting some transactional reward out of it. It’s just something we do.
If someone asks me again, I might just say I’m a kidnapper. They’d probably be less surprised.
p.s. Littlest Loveliest Lab Lemming is half a year old!
Posted by
C W Magee
at
8:39 PM
5
comments
![]()
Labels: Dr. Daddy

We may be working 15 hour days in brutal conditions, but that doesn't necessarily mean that industrial geologists don't occasionally find the time to take a breath and sniff the roses hakeas.
Posted by
Dr. Lemming
at
11:46 AM
1 comments
![]()
Labels: Outback Lemming
I have a new definition for when it is too hot for fieldwork. Last week, the rig broke down, so the other geo and I had a free afternoon. We decided to do some recon in an area of cambrian arkose. The sandstone was somewhat ferruginized, the day was hot, the sky was clear, and there was no wind. About midafternoon, I tried to pick up a rock to get a better look at some stratigraphic features. I failed. I failed because the rock burned my hand. So, my new definition is this: It is too hot to do fieldwork when you can no longer handle the rocks with bare hands.
Posted by
Dr. Lemming
at
11:30 AM
0
comments
![]()
Labels: Daily grind (or polish), Outback Lemming
Browsing through past Wo(G)E entries, it occurred to me that the most ubiquitous geomorphologic feature in the entire solar system had not yet been displayed. So here you go. The lat and lon are insufficient to win this contest; I want the name of the feature as well.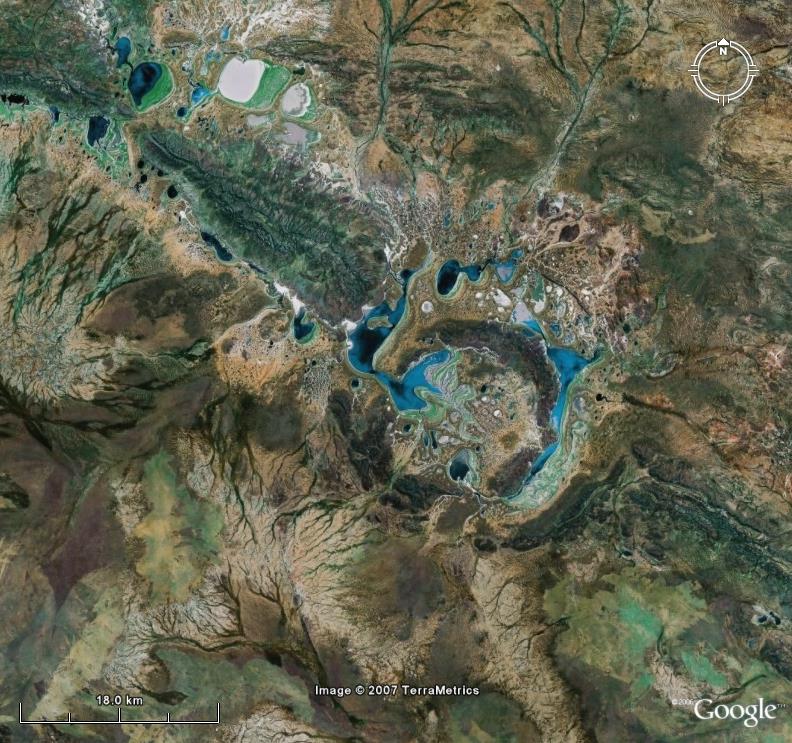
Posted by
Dr. Lemming
at
8:39 PM
7
comments
![]()
Labels: Where on (Google)Earth?
With oil prices flirting with new all-time highs, there has been a lot of talk about the global oil crisis recently. A combination of the price, the peak oil hypothesis, global security issues, and global warming means that the industrialized world’s reliance on petroleum is increasingly being questioned.
One increasingly popular alternative to petroleum is some sort of biofuel; that is oil derived from organisms that have been killed recently, instead of having died millions of years ago. There is a historical precedent for biological oil production; during the first 70 or so years of the industrial revolution, prior to the discovery of petroleum, oil was mainly produced by the whaling industry.
Needless to say, the global thirst for oil has increased since the days of Moby Dick and the old Nantucket sailing ships. But it is still a useful exercise to determine just how many whales would have to be harvested to supply the modern world with oil. In deference to the cultural and literary value of the sperm whale, we will use it as our cetacean of choice for the following calculations.
The current annual production of petroleum is just a shade under 30 billion barrels. Simply dividing this number by the oil that can be produced from each whale should tell us how many whales we will need.
According to industry website SaveTheWhales.org, a sperm whale could produce 2000 gallons, or 47.6 barrels, of oil. Thus a touch of long division tells us that we will need to slaughter approximately 630 million sperm whales each year in order to completely replace our petroleum production. Since there are only an estimated one million sperm whales currently living on Earth, wiping out the entire species would power the global economy for about half a day.
Clearly, a whale breeding program is needed to make such a scheme feasible.
Trouble is, whales are not rabbits. With a slow breeding species like sperm whales, we can only afford to harvest a small percentage- say, 10%, of the total stock each year if we are to use their oil in a sustainable fashion. So in order to have an annual 630 million whale kill, we will actually need a total population of about 6.3 billion animals. That’s about two whales for each person on earth.
And those 6.3 billion whales will get very hungry. Sperm whales have an unusual diet, which consists almost entirely of giant squid. And although the exact rates of consumption are not known, a good ecological rule of thumb for carrying capacities is that predators need a prey population with a body mass that is 10-1000 times larger than that of the predators.
Using a geometric average of 100 times per food chain step, and an average whale mass of 40 metric tones, we will need about 2.5x1016 kilograms of squid to feed our whales. That’s either 840 million billion calamari rings, or a single mollusk that is twice the mass of the Martian moon Phobos. Such a squid is illustrated below.
And because squid are not autotrophic, they too will need something to eat. 2.5x1016 kg of squid will need to eat 2.5x1018 kg of fish, which will need 2.5x1020 kg of zooplankton, which in turn will require 2.5x1022 kg of phytoplankton. This is either an absurd number of microbes, or a giant foram that is one third the size of our moon. And this is where things get tricky.
The Earth’s hydrosphere contains a mere 1.4x1021 kg of water, meaning that it is insufficient to host the phytoplanktonic base of our food chain. Thus we will need to move our whale breeding program to another celestial object, where more water is available. Fortunately, several such objects are available in our solar system.
Ganymede, the mercury-sized moon of Jupiter, has a mass of 1.5x1023 kg, more than half of which is water. Assuming that algal blooms can happily grow at concentrations of 30% of the water mass, this Galilean satellite would be a perfect place to host our whale breeding stock, provided that we can thaw it out. And this should not be too hard to do.
Ganymede, which is gravitationally bound to the giant planet Jupiter, orbits more than 500 million kilometers from the nearest swing electorate. There are relatively few special interest groups or corrupt corporations with a vital interest in the icy satellites of Jupiter, so the only barriers to harnessing this planetoid to fight global warming are physical, not political. Therefore, transporting this enormous iceball to Earth orbit and turning it into a cetacean breeding pool should be substantially easier than producing electric cars, or putting up windmills in Senator Kennedy’s backyard.
I suggest that we simply pop Ganymede into a 1:2 orbital resonance with our current moon. This would give Ganymede an orbital period of two weeks- a useful time frame for the commodities futures market. It would also give us great solar eclipses, and wicked tidal currents. As the whales are needed, they can simply be catapulted into a collision course with Earth, using a trajectory that minimizes the burnup of blubber during re-entry.
While this scheme may seem hare-brained and overly ambitious to some, it is far more practical than taking the bus to work, or driving a Prius.
Posted by
Dr. Lemming
at
10:22 PM
6
comments
![]()
Labels: Greenhouse goofiness
When dropping acid, calcite is a hot, shaken coke, while dolomite is a glass of guiness left on the coffee table over night.
Calcite's reaction to dolomite is simply a matter of age and life experience. Calcite is merely a young, naive carbonate which, due to youth or sheltered lifestyle, has never had a significant relationship with magnesium ions.
Dolomitization is generally a one-way reaction. It is almost unheard of for a dolomite to get calcified.
Posted by
Dr. Lemming
at
5:13 PM
0
comments
![]()
Labels: Underconstrained extrapolations

I had some grad students over for dinner during my last break, and one of them asked me if my field areas were scenic, or just desolate. I wasn’t sure what the difference was, so I figured I’d let y’all make the call. And maybe this guy can tell us how to tell them apart. Whatever you decide, this is where I’ll be for the next fortnight. Catch y’alls later…
Posted by
C W Magee
at
1:22 AM
4
comments
![]()
Labels: Outback Lemming
I recently heard from an old high school buddy with whom I had lost touch for a number of years. She is now an assistant principal at a 6-12 secondary school, and she is currently redesigning their science curriculum. She asked if I had any suggestions, and I told her that I could ask the science blogosphere, and she could stop by the lounge to see what people thought.
Now, I can’t guarantee she’ll actually visit the lounge, much less that she’ll care what any of y’all think. But if any of the scientists who read this e-rag have opinions on secondary school science curricula, feel free to introduce yourself in the comments here and state your opinion. With a little luck, one of the people crafting one such curriculum might possibly take note of what you have to say here.
Otherwise, she’ll have to go with what I told her are the four most important take-home points from the geosciences:
That the Earth is:
-flat
-less than 10 Ka old
-designed by an intelligent creator
and
-warming due to entirely natural causes.
Posted by
C W Magee
at
1:10 AM
5
comments
![]()
Labels: Pompous proclamations
Back in the middle Permian, Astropixie tagged me with the anecdote meme. Now, I lead a pretty boring life, so it has taken me a while to think up some events worth mentioning. And eight is a lot. But I’m leaving soon, so posting a book will give y’all something to doze off while reading for the next few weeks.
1. When we came into the last station on our first field tour of this season, there was a flock of hawks circling the corral. There must have been more than 50 of them, wheeling and soaring over the cattle yards. I’d never seen hawks flock before, and the sight of so many majestic birds was amazing.
The station manager was out, so we asked his partner, who was tending to the garden, what was going on.
“Do you guys have a plague of rodents?”
“No.”
“Did the muster stir up mice or lizards or something.”
“Don’t think so. Why?”
“Well, you’ve got all these hawks up near the yards. We were just wondering if they are after something to eat.”
“Oh, yeah. The boys just castrated this year’s steers, so the hawks are probably looking for…”
We looked up in the sky again. The same hawks were flying. Only now, their curved talons looked more powerful, their hooked beaks crueler, less august. And that glint in their eyes looked considerably more antagonistic. We shifted uncomfortably from foot to foot.
“Look. I think it’s time we moved on up the road…”
2. The Thanksgiving after I finished hiking the Appalachian Trail, my brother and I had an eat-off. We weighed our plates before and after each course of the holiday feast, to determine who was the greater glutton. Although months of binge-and-fast eating had prepared my digestive system for the contest, I chose to rely on strategy as well as bile.
Knowing that my brother did not enjoy eating sweet potatoes, I went for the normal mash early, so that we rapidly demolished that and the turkey. With the mash, bird, and cranberry sauce depleted, I then turned to the orange root, while he was forced to consume Mom’s super filling whole-meal breadrolls. As they were a bit dry, he had to fill precious stomach space with water.
While I won the battle, I lost the war. He continued to grow into his college football O-line body and has soundly eaten me under the table every time since. I think he learned a lesson, though, as he also taught himself to cook, and he is now by far the most accomplished chef in the family.
3. In the second year of my PhD, I went to the Kimberlite Conference in Cape Town, along with several other students in our research group. A couple of us checked out the Lonely Planet guide for things to see. We are all of an age where the anti-apartheid leaders were childhood heroes of ours, and we realized that the diocese over which Archbishop Desmond Tutu presided was near our hostel, and that we would be in town for Easter Sunday.
Figuring that a sermon by one of the great moral crusaders of our time might be worth getting up in the morning for, we set our alarm clocks to hangover-banishing time, became Anglican for the day, and rocked up for the Easter Sunday Service. After doing all the standing up and sitting down whenever everyone else did, we eventually realized that the proceeding were being led by a boring old white guy who bore very little resemblance to the great Archbishop.
The white guy proceeded to give the most boring sermon that I have ever heard in any house of worship on this entire planet. He talked about fidelity to the church, and the important of obedience, and the danger of individuality and of questioning authority. He did not mention the freedom struggle, the new government, or even the historic Good Friday agreement signed three days before.
We stuck out the whole thing, partly because not even I am game enough to walk out of an Easter Service in a strange town, and partly because we clung to the forlorn hope that this guy was the Anglican version of a warm-up act.
It was not until much later, as we medicated the hazardous level of boredom with some beers, that we realized that our guidebook was out of date, and that the Archbishop traded in the preaching gig for the chairmanship of the truth and reconciliation commission several years before.
As we sat in the fine autumn afternoon, we wondered if there might be some sort of lesson in our getting bored out of our skulls by attempting to use a religious holiday to go celebrity spotting. After a few more fine Namibian lagers, though, we stopped wondering what that lesson might be.
4. I have never been overly enthusiastic about formal education. Throughout most of my overly drawn-out academic career, I have received A’s in the classes I liked, and C’s in the ones I didn’t particularly care for. But I didn’t really care one way or the other, with a single exception.
Brown University’s geology department did not offer its own field course when I was an undergrad. Like many east coast schools, it farmed its students out to whatever western course offerings we could get accepted to. I ended up going to Cal State Hayward, partially because it was small and different, but mostly because it was cheap.
As a quiet kid who grew up in a conservative East Coast suburb, and who never lived anywhere more flamboyant than Germany, Northern California was a bit of a shock to the system. Reticent around groups of strangers at the best of times, I simply slunk into the back corner of the classroom for orientation, determined to keep my own company.
The local students knew each other already, of course, and the professor gave th5e rest of us a short introduction, even though most of the other students had taken classes with the Hayward mob before, and only two of us were from East of the Mississippi.
Finally, the professor said who I was, and where I was from, and that was all OK. Except that he then continued….
“Now Chuck here is a bit of an unusual situation. We actually had more people apply to this course than we had places, so we had to turn a few people away, and Chuck was missing several pre-requisites for this class. But I thought his application essay was somewhat amusing, and if we’re all going to stay sane for the next six weeks, we’re gonna need some comic relief. So, I decided to bring him along. As for the prereqs, since this class is graded on a curve, you can all consider his inclusion a gift to your grades.
I was absolutely stunned. Nobody had asked me to entertain anyone ever before, much less an entire crew from whom I could not escape. And the curve comment was outright insulting. As I sat there, trying to figure out how to get my jaw back up to my mouth, only one thought registered in my mind. I HAD to get an A in that course.
In the first field area, I pushed so hard that my partner got exhaustion, slipped in an Arroyo, and busted several ribs. This cut into our mapping time, and my grade for that project. But after that I started to get canny instead of simply powering through, and I earned that A by the end of the course.
It was not until years later that I realized the professor had read me like a book.
5. When I first came to Australia, I tricked myself into thinking I was prepared by looking up Australian slang on the internet. Nothing I ever saw said anything about garnishes, so when it came my turn to cook in the share house I was in, I pulled out my American lasagna recipe and headed to the store. Once at the supermarket, I though nothing of grabbing a big jug of tomato sauce, oblivious to the possibility that it could be anything other than sauced tomatoes.
We sat down to eat, and after a bite I realized that something was amiss. The cheeses were blended fine, the pasta was well cooked, but the sauce didn’t seem quite right. It was unusually sweet, and extremely vinegary. Despite these obvious warning signals, I puzzled over the strange taste for several days, before a few chats with my native housemates and a good hard look at the ingredient list finally convinced me of what should have been obvious all along; in Australia, tomato sauce is the substance that American call Ketchup.
The really scary thing, however, is that my housemates said the lasagna tasted great.
6. I was a post doc in Washington DC during the sniper attacks in 2002. I suppose that in the long run it was good for me in that my cycle commute times dropped by 5-10 minutes for the duration, improving my fitness levels, but it was stressful at the time. I really wanted to let off some steam by calling up a radio station, and request Pat Benatar’s Greatest Hit with a dedication to the scumbag who was going around shooting up parking lots. But I was too cowardly, disorganized, and lazy to follow through with that plan.
7. I love hitch hiking. I’ve thumbed down rides of up to 200 km on 4 different continents. The people who give rides always have amazing stories, as boring folks generally don’t slow down. One of the oddest rides I scored, however, was more of a business transaction than a hitch.
I was in Idaho Falls in the summer of ’94, seeing the west and getting dissed by girls in the weeks between field school and the start of fall semester. And I was in a bit of a fix.
Greyhound had previously lost everything I owned save my wallet, my journal, and half a loaf of banana bread. More seriously, my grandfather had unexpectedly passed away back east, so I needed to catch a flight leaving from Jackson WY the next day. I decided to start hoofing it, realizing that my luck might be better the farther out of town I got. While walking, I came across a woman broken down on the side of the road. She had a flat, and didn’t seem real handy with a jack and a tire iron, so I bartered a repair for a lift to the Jackson Pass junction. Despite her not being a lift-giving regular, we engaged in the ritual chit-chat anyway, and it eventually came out that I was a geology student. This piqued her interest.
“Is it true,” she asked, “That the states of California, Oregon, Washington, and Idaho are going to fall into the ocean in 1998?”
It took every ounce of self control I had (e.g. both of them) not to say, “That’s right, ma’am. We’re all going to die.”
I haven’t used my thumb in anger since getting married, aside from an emergency situation on the honeymoon. It is one of those things where I don’t realize what I’m missing until sitting down and reminiscing. But these days, I wouldn’t hitch with the baby anyway- driving a bub without a properly installed car seat is just too risky.
So, that’s seven. One anecdote to go. So in order to liven this place up a bit while I’m out, I’m gonna do something foolhardy. I know there are people- people from my real life- who occasionally read this blog. Most of these folks are generally fairly shy when it comes to commenting. But all of them have at least a little dirt on me. So here is what I will do. I’m opening up anecdote #8 to the readership. If you have some illuminating, embarrassing, or remarkable occurrence that you’d like to share, post it in comments below. I’m off tomorrow, so I can’t even screen the comments or defend myself.
Posted by
C W Magee
at
12:42 AM
2
comments
![]()
Labels: Irreproducible idiocy
There have been a lot of things written about Alice Springs over the years. However, despite the many descriptions and opinions of the desert atmosphere, the scenery, the culture, the art, and the politics, the town’s ductile deformation is rarely mentioned. Which is too bad.
Look at the above rock, from an outcrop on Bath Street. Viewed end-on, it could almost be mistaken for a granite porphyry. But a simple rotation out of the plunge direction shows extensive linear deformation. With rocks like this, who needs camel races?
Posted by
C W Magee
at
10:03 PM
1 comments
![]()
Labels: Outback Lemming
Starting about a year ago, I have seen the above slogan used increasingly in zinc-bearing goods, especially batteries made in China. It is one of those statements that is both technically correct, extremely misleading and dangerously deceptive.
The fact of the matter is that nobody ever intentionally added mercury to zinc products. This is why I have no doubt that the statement is correct. This is also what makes it misleading. The statement implies that Hg contamination is caused by an additive, kinda like lead in gasoline. But that implication misrepresents the most obvious source of mercury contamination.
A quick look at a periodic table will show that Zn, Cd, and Hg are all in column 2b, and one might surmise from this that they have broadly similar geochemistry. And indeed they do. In nature, they all occur primarily as +2 chalcophile cations, meaning that sulfur, and not oxygen, is their preferred anion in the Earth’s crust. Indeed, most zinc mined today is mined from sphalerite (ZnS) ore. But because of the similar chemistry of these three elements, Cd and Hg can, and generally do substitute for Zn in natural sphalerite to varying extents, depending on the particular mine and type of deposit. So in order to have zinc that is contaminated with Cd and Hg, you don’t need to add those toxic metals- they are often already in the orebody.
Therefore, the important health and safety question is not whether or not Hg has been added, it is whether or not the primordial Hg already present in the ore has been removed during the smelting and refining process. Most of the products I’ve seen with the “No mercury added” claim also say “Made in China” , a country not exactly famous for stringent environmental regulation of its heavy industry. So the fact that they put a true but irrelevant statement on their product does not exactly convince me that it is non-toxic.
In fact, it might be a fun undergraduate research project to figure out just how much Cd and Hg various zinc-based “no mercury added” products actually contain. Too bad I don’t work in a lab anymore…
Posted by
Dr. Lemming
at
6:42 PM
5
comments
![]()
...is shorter than this blog entry.
Posted by
Dr. Lemming
at
9:17 PM
0
comments
![]()
Labels: Outback Lemming
Greetings from Alice Springs.
62 Years ago today, the first atomic bomb was detonated in anger.
I'm heading off into the desert to look for uranium.
Go figure.
Posted by
C W Magee
at
4:13 PM
0
comments
![]()
I'll be in my office.*
* Office: place with no paperwork, no furniture, no books, no walls, no running water, no electricity, no surface water, no roads, and no people; the place I must go to earn my paycheck.
p.s. anyone getting restless is encouraged to try their hand at Where on (Google) Earth number 38.
Posted by
C W Magee
at
1:08 AM
0
comments
![]()
Labels: Daily grind (or polish), Outback Lemming
I haven’t written much about actual lab science recently, and Dr. Carl Spandler, a former postdoc of ours at the ANU, recently published a funky nature paper using data from our laser lab, so here’s a summary of what they did. I should point out that I didn’t do any of the gruntwork for this research. I did, however, provide some minor assistance for some of the followup studies.
A summary of the paper, and the abstract are free. The full paper requires a subscription.
But first, a description of melt inclusions.
As a magma (or silicate melt) cools, it starts to crystallize. In the case of basalt, the first crystals to form are generally olivine. Sometimes, as these crystals are growing, they grow in a geometry that allows a blob of the melt from which they grow to become trapped as an inclusion inside of the crystal. If the crystal is then transported somewhere else, it keeps this entombed blob of melt.
Sometimes, melt inclusions are anomalous. An anomalous melt inclusion in any melt inclusion with a trace element composition different to what one would expect to find. One trace elemental component that is often anomalous is the relative abundances of the lanthanides, known to geologists as the rare earth elements. For those who can’t remember the names of these elements, a mnemonic is available here.
In the Earth’s mantle, rare earth elements all generally occur as trivalent oxides in melts, silicates, or (rarely) phosphates. Because they have the same charge, similar oxygen affinity, and gradually decreasing ionic radius, their behavior in geochemical systems relative to each other can be predicted fairly well. Thus, deviations in the relative abundances of REE’s compared to a particular reference value are used to infer all sorts of geological stories.
Many anomalous melt inclusions have anomalous REE patterns, and these have been used to constrain the origin of these melt inclusions. For example, the heavy REE have a small enough ionic radius to easily fit into the crystal structure of garnet, while the light REE do not. So if a rock containing garnet partially melts, and that melt is in equilibrium with some residual garnet, then the melt will have a very high La/Lu ratio, because some of the rock’s Lu will stay behind in the garnet, while none of the La will.
Because garnet is only stable in the mantle at high pressures, a “garnet signature” REE pattern can be used to infer a deep source of melting- and most basalts do not show significant garnet signatures. A melt inclusion with a garnet REE signature in a rock with no bulk garnet signature would be said to be anomalous.
Of course, in order to be geologically meaningful, the REE in a melt inclusion have to be effectively trapped by the crystal. If the temperatures and residence times are too large, then solid state diffusion might allow the melt inclusion to equilibrate with the melt outside of the crystal. While most melt inclusion people have previously assumed that the existence of melt inclusions requires them to not re-equilibrate, the purpose of the experiments presented in the Spandler et al. paper was to determine whether or not REE diffusion can occur in typical magmatic systems.
So, this is what they did:
1. Get a population of normal MORB melt inclusions that were unlikely to have any anomalous inclusions in them.
2. Determine the temperature at which these inclusions were trapped in the host olivine.
3. Determine the composition of the olivine that traps the inclusions.
4. Calculate what the composition of a basalt should be, in order to be in equilibrium with the olivine at the trapping temperature of the inclusions, under a fixed fO2 and atmospheric pressure.
5. synthesize a basalt of that composition. A synthetic basalt made from lab reagents will have no REE in it.
6. Dope the synthetic basalt with several hundred ppm of the following REE: Pr, Eu, Tb, Ho, Lu
Presumably these were chosen for the following reasons: AS odd-numbered elements, they have lower abundance, so the ration of synthetic to natural is greater for a fixed concentration. Also, the detection limits and counting stats for the mass spec are better, because all but Eu are monoisotopic clear mass numbers, so you can count all the ions, not just those from a minor isotope.
7. Heat the synthetic basalt up to the trapping temperature, toss in the intact olivines containing natural melt inclusions, and let them sit for varying time periods.
8. Quench, extract the olivines, polish them down to expose the melt inclusions, and see if any of the doped elements diffused into the melt inclusions.
Not only did they find that diffusion occurred, but they were able to determine what the diffusion coefficients were. And applying those coefficients to magmatic systems showed that REE will diffusively re-equilibrate on a timescale of years. Short-lived nuclide and geophysical constraints suggest it takes thousands to tens of thousands of years for melts to migrate from their mantle sources to the surface. Thus, anomalous melt inclusions must be trapped in a late stage of magma migration, as any melt inclusion captured early on would re-equilibrate long before it was erupted the surface.
While the paper was languishing in review, Carl described the results in a talk at Goldschmidt. It was that talk that caused Al Hoffman to blow his top, which was highly entertaining for us pudknockers in the back row. But melt inclusion research is an incredibly finicky and laborious line of study, so I can see how being shown that it can’t possibly mean what you think it means could be upsetting.
Anyway, that’s the lab denizen’s view of the study. It would be interesting to see what a skeptical petrologist makes of it.
C. Spandler, H. St C. O'Neill & V. S. Kamenetsky 2007. Survival times of anomalous melt inclusions from element diffusion in olivine and chromite. Nature 447, 303-306
Posted by
C W Magee
at
1:37 AM
0
comments
![]()
Labels: REE
Sciencewoman is starting a new job, looking for childcare, and trying to adjust to a cross-country move using a moving company that operates using the geologic time scale. If that isn't enough, the IRS has issues with her taxes, her mom is sick, and some random numbskull who doesn't know his ass from a hole in the ground is whinging about her ability to nurture her beautiful baby. I'm starting to wonder if her new home town is Nashville, and if her dog and her truck are looking over their respective shoulders...
But the fact of the matter is that her down-to-earth style, scientific outlook, and extraordinary personal honesty make her one of the best science bloggers on the web, so if any of y'all wanna pop over to her place and write an encouraging word, I reckon she'd appreciate it.
Posted by
C W Magee
at
1:00 AM
1 comments
![]()
Labels: Worth leaving the lounge
I while back I mentioned that Mrs. Lemming, Littlest Loveliest Lab Lemming, and I were trying a variety of different nappy types. For the first 11 weeks or so of her life, LLLL used a couple of different reusable nappies and disposables.
The first sort of nappy that we used on our daughter was the standard, terry-towel cloth nappy. This is the chondrite of diapers, an undifferentiated square of cloth with no functional adaptations. The hospital where LLLL was born provides only cloth nappies, and encourages new parents to also use them at least until the meconium changes and any potential jaundice passes. I think the idea is that it is easier to count and keep track of eliminations if they aren’t all sucked into the silica gel of doom.
Cloth nappies were fine for those first few days, but after the first week they started suffering containment failures, and the size we had at home was too big anyway.
For the next 10 weeks, we used two other nappy options on our daughter. After 11 weeks, she grew out of newborn sizes, so everything changed.
The disposable option was huggies. Everyone we talked to said that they had the best containment, and we found that the newborn size worked quite well.
The reusable nappies were bummi wraps from Green Mountain Diapers in the US. These consist of a water resistant nylon outer wrap which holds a cloth diaper in the appropriate position. With urination and small poos, only the cloth insert needs to be changed, while with large poos everything gets messy.
Both systems leaked about once a week or so, and the reusables generally soiled the (inside of) the outer wrap one or two times a day. For the first month, we alternated each system for a few days at a time, because using either all disposables or all reusables for more than a few days gave her nappy rash, which changing systems got rid of.
After the nappy rash past, we usually used the reusable nappies at home and the disposables for going out, or for overnight. In terms of grossness, time consumption, or difficulty I don’t think there is that much difference, except for doing the extra load of washing, which takes a while. The reusable covers are definitely cuter, but since it is the middle of winter they aren’t often visable.
During my last week of paternity leave I considered calculating a partitioning coefficient between bottom and nappy for both types, but sleep deprivation and inconsistent fecal texture stymied this project.
In terms of waste, the disposables generally meant one more trash bag per day*, while the reusables meant an extra load of washing every day or two. We wash the nappies in the washing machine on the “biologicals” cycle, with an additional vinegar rise cycle afterwards.
After 11 weeks, LLLL grew out of the infant size and also changed from 4 medium poos per day to one megashit every 24-36 hours. We found the infant sized female huggies and baby love disposables to be fairly ineffective at containing megashits, but the mamia brand from Aldi seems to be doing OK. The reusables contain well, but the whole package needs to be changed, not just the insert.
Did anyone have any particular questions about the advantages or disadvantages of one system over the other?
* We use plastic grocery bags as our garbage bags, and filled 2-3 per week before the baby.
Posted by
C W Magee
at
11:31 PM
1 comments
![]()
Labels: After-hours analysis, Dr. Daddy
I usually don’t bother with antiscience. It’s kinda like researching and dobbing in companies that advertise Mexican sex drugs- debunking takes way more effort than spewing crap, so it is hard to win. But Sabine pointed me to a recent article on abiotic oil that is a textbook case of argument from ignorance.
An argument from ignorance generally works like this:
1: “I have no idea how much I don’t know. In fact, I think I know everything worth knowing.”
2: “I have no understanding of X, and since I know everything worth knowing, X can’t be important or correct”
3: “Because X is not important or correct, any arguments based on X must be irrelevant or wrong.”
In this article, we have:
“Kerogen, it turns out, is not a chemist's term. Kerogen is a loose, geological term…”
“Kerogen is not a term typically found in chemistry textbooks or specifically used by professional chemists. Use of the term kerogen is generally a signal that you are dealing with a petroleum geologist or engineer, not a chemical scientist.”
“We have yet to find a chemistry textbook that refers to "kerogen" or describes any combination of ancient algae, tiny Mesozoic sea animals, or dinosaurs as necessary or sufficient ingredients in the formation of common saturated hydrocarbons such as methane, ethane, propane or butane.”
“The observation of methane formation at mantle pressures is significant because it demonstrates the existence of abiogenic pathways for the formation of hydrocarbons in the Earth's interior”
“Has anyone ever taken a flask of downed flora or dead protoplasm and produced a hydrocarbon fuel out of the mixture, or is this a process for alchemy?”
Posted by
C W Magee
at
12:15 AM
2
comments
![]()
There’s a great new blog over at sciborgs, where the Hoofnagle brothers describe the psychology and tactics of denialist argument. I think that their main aim is the funded disinformation campaigns of interest groups and extremists, but I am beginning to suspect that the underlying psychological basis for inventing justifications is more widespread.
For example, the denial tactics used against global warming research are familiar to everyone who has worked in Earth sciences, and the denialism blog describes these well. But recently, I have seen the same pseudo-arguments appear in an entirely different context.
The institution I recently left has a terrible record at hiring women for senior positions (e.g. mass extinctions and Wilson cycles are more common). And it seems that recently, faculty has actually realized this, and decided to think about what, if anything, should be done. But although nobody has come out and said that the school is better off with 100% male professors and 60% female students, there have been a number of arguments against action that could have been torn right out of a coal lobbyist’s play book, or the denialism deck of cards.
Examples:
Too expensive: We don’t have the money to hire anyone, so we’ll have to wait and see (3 spades).
Too damaging to (scientific/economic) output (6 hearts): Hiring the best women instead of the best people means lowering standards (and thus, presumably, quality of life).
Too daunting: the existing faculty makeup simply can’t be rebuilt overnight.
Wait and see (3 spades): The recent increase in female geoscience participation just needs time to work its way through the system. There is no problem (2 clubs), we just need to let time sort itself out.
Further study (5 spades). I think they wanted to interview all female alums to see what turned all of them off. Dunno if that actually went ahead, though.
Mere inconvenience (2 spades), no harm (3 hearts), no problem (2 club): Thankfully, nobody has mentioned these yet, so at least there is progress.
Posted by
C W Magee
at
12:49 AM
2
comments
![]()
Labels: Scientific hoop-jumping
(Simul-blogged with Molecule of the Day.)
Much of our exploration area is loacated in gidgee country. Gidgee is a slow growing acacia tree that forms scrub, savanna, or low woodland in the claypans, stream beds, and dolomite areas of the basin.
From the bushcraft point of view, gidgee is the perfect firewood. It burns easily, hot, and long, with few sparks or pops. It was not unusual for us to put a log on at dinner and use the coals to heat the billy for coffee water the next morning. Cattlemen, however, are less fond of the tree.
At certain times of the year, or in periods of stress, Acacia georgina can contain high levels of fluoroacetic acid, the sodium salt of which is the poison 1080. Molecule of the Day gives more information on the chemistry and toxicity of this substance here, but the effect in the field is that cattle with gidgee poisoning are very prone to stress, and can drop dead when startled or disturbed. Annual cattle losses in the NT from gidgee are on the order of a couple million a year, and there are stories of helicopter-based geophysical surveys wiping out small herds just by overflying them. This is one reason that cattlemen don’t like helicopter work.
The other reason that cattlemen don’t like helicopter work is that healthy normal cows in eventually get used to overflights after a little while. This is great for the cows, but it is not ideal for the station manager when he plonks down several thousand bucks to rent a chopper for muster. The acclimated cows won’t run from a mustering chopper. They’ll just look at it, look at each other, and go back to eating the grass around the gidgee trees.
Posted by
C W Magee
at
7:30 AM
0
comments
![]()
Labels: Outback Lemming
In the outback, a vehicle is important for survival. Every summer there are tales of tourists who expire after leaving their vehicle in order to walk for help, without appreciating how far from anywhere they are, or how fast they dehydrate under the Australian sun. While it is important to stay with a vehicle if it breaks down, better yet is to not break down in the first place, or to carry supplies and know how to fix the problem. Therefore, I recommend that any traveler prevent his vehicle from degenerating into this sort of condition (click image for larger version):
Note that the operational status of this particular vehicle has been concisely described by somebody with a cutting torch. If the first couple letters of said description are not easily legible in this photo, try reading the antishadow in the wheel well instead.
Posted by
C W Magee
at
12:21 AM
1 comments
![]()
Labels: Outback Lemming

This has to be the easiest woge since the LGM ice caps slid into the sea. While the Schott Rule still applies (one hour per previous win), people waiting for the clock are welcome to name as many glacial features as they can make out in the picture.
Kent got it last night, bit I'll put up Yami's clues anyway, since they are interesting.
Oblique Google Earth view, looking south from small western plateau:
Actual view from same location:
Posted by
C W Magee
at
9:54 AM
8
comments
![]()
Labels: Where on (Google)Earth?
By far the hardest part of the new job is that it requires me to be away from my wife and daughter for extended periods of time. In fact, this last trip ended up being something like 20% of LLLL’s entire life thusfar. And there were plenty of changes to be observed when I got back.
First of all, she is huge. Her head was bigger, and although her hair was longer, it seemed to give a similar amount of coverage. From this observation, I surmise that her rate of hair growth is the square of the rate of head circumference increase, assuming a constant number of follicles.
Her cries developed syllables. This must be the beginning of speech.
She went up a nappy size, and has transitioned into larger, less frequent poos (Mrs. Lemming gave me the opportunity to change a nappy in the airport change room immediately after getting off the plane).
She smiles much more convincingly, and laughs, and gurgles.
Despite all the changes, she is just as cute as ever.
Posted by
C W Magee
at
8:13 AM
0
comments
![]()
Labels: Dr. Daddy
The state government of Western Australia is currently offering skilled migration visas to geologists and geophysicists in order to meet the skills shortage caused by the continuing resource boom. The STNI scheme is a two year working visa, successful completion of which culminates in permanent residency. Applicants under this scheme must live in Western Australia and work for a WA company. Details on the visa are found here. The deadline for the current visa conditions is 17 August 2007.
Needless to say, most of the earth science job shortages are in the minerals industry. However, defection of people from other geoscience areas (e.g. people like me) means that there may be other types of employment available. You won’t know until you look. So if you are a geologist or geophysicist, you want to try the Australian lifestyle, and you meet the criteria (see link), check it out.
Note: I am not a migration agent. I strongly recommend that anyone interested in these schemes contact a professional for accurate information on how they work.
Posted by
C W Magee
at
9:31 PM
0
comments
![]()
Labels: Just sayin', Tricks for young players, Worth leaving the lounge
I can't upload images larger than about 150k, even though available space is huge. I seen to have the same limit on gmail attachments. If any of y'all have seen this problem, or know a solution, please tip me off so that I can show off field pics...
Posted by
C W Magee
at
11:21 PM
4
comments
![]()
Can you imagine defining targets in this sort of terrain without it?
p.s. Apologies to any Midwesterners who get homesick from the relief in this terrain.
Posted by
C W Magee
at
10:39 PM
2
comments
![]()
Labels: Daily grind (or polish)
There's nothing like winding down after a field posting by knocking off a woge as a distraction from trying to find a route that doesn't bog the truck so much. So for everyone back on break, here's another picture of our beautiful planet. The geological theme for this episode is structurally controlled drainage. The environmental theme is clearcutting.
In order to open the contest up a bit, I will ask previous winners to wait 24 hours before answering.
Posted by
Dr. Lemming
at
11:38 PM
10
comments
![]()
Labels: Where on (Google)Earth?
Hopefully the next line of the song will not apply...
p.s. I apologise for the low image quality, but blogger won't take anything over 200 kb for some reason...
Posted by
Dr. Lemming
at
10:42 PM
0
comments
![]()
Via A K8, a Cat, a mission, Select smart.
What we have here is a C- from the fruitcake parties, a D and a D- from the Republicans, and a D- who isn't even running from the Dems. Then there's a whole bunch of failures. I'm looking forward to this election.
Apologies for formatting snafus.
Your Results:
| 1. | Theoretical Ideal Candidate (100%) | |
| 2. | Kent McManigal (71%) Click here for info | |
| 3. | John McCain (66%) Click here for info | |
| 4. | Ron Paul (62%) Click here for info | |
| 5. | Al Gore (60%) Click here for info | |
| 6. | Bill Richardson (55%) Click here for info | |
| 7. | Joseph Biden (55%) Click here for info | |
| 8. | Mitt Romney (55%) Click here for info | |
| 9. | Rudolph Giuliani (52%) Click here for info | |
| 10. | Wesley Clark (52%) Click here for info | |
| 11. | Newt Gingrich (51%) Click here for info | |
| 12. | Tom Tancredo (51%) Click here for info | |
| 13. | Barack Obama (50%) Click here for info | |
| 14. | Chuck Hagel (50%) Click here for info | |
| 15. | Hillary Clinton (50%) Click here for info | |
| 16. | Dennis Kucinich (49%) Click here for info | |
| 17. | Fred Thompson (48%) Click here for info | |
| 18. | Tommy Thompson (48%) Click here for info | |
| 19. | Christopher Dodd (47%) Click here for info | |
| 20. | Sam Brownback (44%) Click here for info | |
| 21. | Duncan Hunter (43%) Click here for info | |
| 22. | John Edwards (39%) Click here for info | |
| 23. | Mike Huckabee (37%) Click here for info | |
| 24. | Mike Gravel (36%) Click here for info | |
| 25. | Jim Gilmore (32%) Click here for info | |
| 26. | Alan Augustson (31%) Click here for info | |
| 27. | Elaine Brown (8%) Click here for info |
Posted by
C W Magee
at
11:02 AM
2
comments
![]()
Labels: Just sayin'