I’m off for summer holidays- so see all you folks in January. I'll come back when I grow weary of beautiful beaches and summer fun. A few parting points:
Before your Christmas celebrations get too involved, please spare a kind word for Sabine and her dog Griff. Griff was shot and wounded by a depraved redneck grinch four days before Christmas. I hope he gets well soon.
And now, the merriment.
Announcements:
The writers of Inky Circus have given us a new science magazine, Inkling. Move over Seed, there's a new game in town. I'll even forgive their starting the subtitle with a preposition.
If I ever get sick of measuring stuff, I might have a new career as an SEM model. I was filmed acquiring backscatter images of either Antarctic sphenes or Brazilian monazites while over at biology earlier this month. Why so few actual biologists are in these pictures makes me somewhat suspicious.
Holiday cooking:
Brownies, by Miss Prism
Margarita meringue, by Yami
My Pecan Pie
Apple Pie by Clifford
Tapioca and eggnog, by Sara
If I’ve missed your holiday recipe, I apologize. Post a link in comments.
I'm a geochemist. My main interest is in-situ mass spectrometry, but I have a soft spot in my heart for thermodynamics, poetry, drillers, trees, bicycles, and cosmochemistry.
Friday, December 22, 2006
Luke-warm lava
Today, after a couple of false starts, I managed to finally reduce some data that’s been hanging over my head for a few months. I generally don’t do much data reduction or interpretation- I get people set up and collecting, and leave the minor step of interpretation up to them. It is only the really strange, unusual, or perverse projects that get kicked down the chain of command into my lap. Luckily, this was a project that actually interests me, as opposed to the completely bizarre, esoteric stuff that sometimes comes up.
So, I finally finished my crunching, fed the geochemistry into a geothermometer to get a crystallization temperature, and scratched my chin over the answer. 90 degrees. Not 900 degrees, 90. The temperature of a cup of coffee. Trouble was, this was not a sugar crystal. So the implications were somewhat interesting.
Let’s just assume that both my measurements and the thermometer (calibrated for a completely different range) were correct. Imagine, if you will, what a world we would live in if magmatic temperatures were below the boiling point. Better yet, imagine a world in which volcanoes simply erupted coffee. Some key advantages:
-Hawaiians would be more edgy, less laid back.
-The people of Martinique would still be alive today, as the 1902 eruption would have simply covered St. Pierre in a dollop of foamy milk, instead of destroying the city with a nuée ardente.
-The mud pots of New Zealand and Yellowstone wouldn’t need a separate coffee store.
-Seattle could still be the retail coffee capital of the world.
-My data might actually make sense.
This might seem like a bit of a stretch, but remember, it is the season of forgiveness. Imagine a better world, a kinder world, where natural disasters are after-dinner beverages. The sugarplum fairies could transform the bushfire embers into snowflakes and candycanes. We could have joy for all, and peace on earth. And if you can’t imagine that for Christmas, then what future is there? A role in a Dr. Suess book? Or perhaps the life of a Dickensian mench, cruelly ignoring the crippling influence of ignorance and want. So Merry Christmas everyone, and have a happy New Year.
So, I finally finished my crunching, fed the geochemistry into a geothermometer to get a crystallization temperature, and scratched my chin over the answer. 90 degrees. Not 900 degrees, 90. The temperature of a cup of coffee. Trouble was, this was not a sugar crystal. So the implications were somewhat interesting.
Let’s just assume that both my measurements and the thermometer (calibrated for a completely different range) were correct. Imagine, if you will, what a world we would live in if magmatic temperatures were below the boiling point. Better yet, imagine a world in which volcanoes simply erupted coffee. Some key advantages:
-Hawaiians would be more edgy, less laid back.
-The people of Martinique would still be alive today, as the 1902 eruption would have simply covered St. Pierre in a dollop of foamy milk, instead of destroying the city with a nuée ardente.
-The mud pots of New Zealand and Yellowstone wouldn’t need a separate coffee store.
-Seattle could still be the retail coffee capital of the world.
-My data might actually make sense.
This might seem like a bit of a stretch, but remember, it is the season of forgiveness. Imagine a better world, a kinder world, where natural disasters are after-dinner beverages. The sugarplum fairies could transform the bushfire embers into snowflakes and candycanes. We could have joy for all, and peace on earth. And if you can’t imagine that for Christmas, then what future is there? A role in a Dr. Suess book? Or perhaps the life of a Dickensian mench, cruelly ignoring the crippling influence of ignorance and want. So Merry Christmas everyone, and have a happy New Year.
Thursday, December 21, 2006
Google simply doesn’t give a shit about woman scientists’ publication records
Back in September, I wrote about how academic indices are incapable of realizing that a woman researcher who changes her name is still the same person. As a test name, I used my grad school colleague Helen (she has published under both names, and when I asked her, she said she didn’t mind me performing this experiment). In September’s typically bombastic, tongue-and-cheek post, I gave a couple of rather snide hypotheses which I could pretend to address with symbolic examples instead of rigorous testing.
I would like to revise those hypotheses, at least with respect to Google Scholar.
I now suggest that Google Scholar does not realize that Helen Degeling and Helen Tomkins is the same researcher because:
Hypothesis 1: The google search technology is not sufficiently savvy to determine that she is one person who changed her name.
Hypothesis 2: They have the technology, they just can’t be bothered to apply it to this particular issue (e.g. they don’t give a shit).
Hypothesis one is testable. All we need to do is to determine if any of the scientific literature accessible via Google Scholar turns up evidence connecting these two Helens. If no such evidence exists, then it would be fair to state that Google Scholar, as currently programmed, simply doesn’t know that Degeling became Tomkins.
The easiest way to do this is to search for “nee Degeling.” Here are the results.
Those of you with journal access can see that “Helen Tomkins (neé Degeling)” is listed in the acknowledgements, and that Google scholar is smart enough to have found it there (which is why Watson et al. turned up in this search).
Thus hypothesis 1 is refuted. Of the hundreds of articles published in Helen's field this year, Google Scholar unerringly picked out the one that connects her maiden and married names. It just doesn’t bother to use this information when asked to compile her publications. Thus hypothesis 2 becomes our best working hypothesis, given the available data.
Suppositions as to why Google doesn’t give a shit are left as an exercise to the reader.
I would like to revise those hypotheses, at least with respect to Google Scholar.
I now suggest that Google Scholar does not realize that Helen Degeling and Helen Tomkins is the same researcher because:
Hypothesis 1: The google search technology is not sufficiently savvy to determine that she is one person who changed her name.
Hypothesis 2: They have the technology, they just can’t be bothered to apply it to this particular issue (e.g. they don’t give a shit).
Hypothesis one is testable. All we need to do is to determine if any of the scientific literature accessible via Google Scholar turns up evidence connecting these two Helens. If no such evidence exists, then it would be fair to state that Google Scholar, as currently programmed, simply doesn’t know that Degeling became Tomkins.
The easiest way to do this is to search for “nee Degeling.” Here are the results.
Those of you with journal access can see that “Helen Tomkins (neé Degeling)” is listed in the acknowledgements, and that Google scholar is smart enough to have found it there (which is why Watson et al. turned up in this search).
Thus hypothesis 1 is refuted. Of the hundreds of articles published in Helen's field this year, Google Scholar unerringly picked out the one that connects her maiden and married names. It just doesn’t bother to use this information when asked to compile her publications. Thus hypothesis 2 becomes our best working hypothesis, given the available data.
Suppositions as to why Google doesn’t give a shit are left as an exercise to the reader.
Tuesday, December 19, 2006
Thermodynamics of hot chicks
Over in Cocktail Party Physics, Jennifer has been complaining about boys’ propensity to measure their self-worth by the heat of their girlfriends. Meanwhile, Janet, Tara, and their commenters seem to be confused about how the heat of a chickybabe can be properly and scientifically defined.
The definition, quantification, and calculation of heat was one of the most enduring and important scientific discoveries of the 19th century. For those unfamiliar with it, I will provide a brief historical synopsis of the quantification of hot chicks, and the development of the theory with which this value, Q, can be calculated. Finally, by using Gibbs' free energy, I will explain why young males are so insistent on maximizing the Q of their girlfriends.
The first attempts to rigorously define female attractiveness were done by the well known, and heavily idolized, Heat Engine. Heat Engine was a 19th century boy band with the following members:
Like most boy band members, these four engineers possessed limited musical skill, and vast teenage lust. However, because lip-synching had not been invented in the 19th century, the boyz had to find a different mechanism with which to reconcile their talent with their testosterone. This is why they invented thermodynamics.
The original thermodynamic equations and models were invented to explain the inefficiencies inherent in translating musical prowess into sexual activity. In his seminalpaper single Réflexions sur la puissance motrice du feu( in English, How to get laid without playing an encore), Sadi Carnot proved that there was a maximum theoretical efficiency- about 40%- for the procurement of sex via musical activity. While this seemed fairly alarming given their meager talent, it was actually a ten-fold increase on the hottest touring bands of the time.
Although that hit single was enough to immortalize Heat Engine, Rudolf Clausius one-upped his lead vocalist by composing theformula album
dQ=dW+dE
In otherwords, the change in heat equals the work done plus the change in energy. This allowed mathematical confirmation of the empirical observation that hardworking, energetic chicks were hot. This simple, wholesome German formulation attracted little notice. However, his followup formulation, dQ=TdS, was considered obscene, and fell off the charts in three weeks.
Clausius and Emile Clapeyron went on to produce many successful duets on numerous seductive properties. These included the platinum selling “Second law of thermodynamics”, the irreversibly splendid “Entropy”, and an equation, named after themselves, that described the energy spent and entropy gained in changing phase or shedding clothes. They then tackled the thermodynamic concept that broke up the band, adiabatic cooling.
Carnot insisted that when a body expanded, she became less hot, a process that he described as adiabatic cooling. While the other three were in a jam session working out the instrumentals for this theory, he leaked it to the public, thus creating the multi-million dollar fashion, diet, and beauty industries, all based on the principle that denser is hotter.
Helmholtz and Clausius were both married to stout German hausfrauen by this time. When they found out about Carnot’s betrayal, they got all loga-rhythmic on his ass. In a blistering rush of calculus and musical genius, they proved that despite the change in temperature, adiabats not only conserved heat, but were also isentropic, and therefore reversible. Alas, the same did not hold for Carnot’s career, and he was banished from the band, dying from an STD on the streets of Paris later that year.
Unfortunately, by that time the myth of adiabatic cooling was firmly entrenched in the public imagination, and even a lurid refutation was not enough to change public opinion. The German prediction of heat conservation was confirmed near the end of the century in a shocking thermodynamic experiment. A deranged Italian highway engineer tried to increase the heat of half a dozen schoolgirls, by attempting to adiabatically compress them. Unfortunately, he used a steamroller to do so, which resulted in an irreversible, entropy-gaining densification to cardboard thickness. However, in the enquiry that followed, it was shown that the highly compressed corpses were in fact no hotter that the original schoolgirls had been before the attack. Sadly, the thermodynamic implication of this case was not one of the angles most heavily featured in the tabloids, so the myth survived unscathed.
Back in the 19th century, with Heat Engine’s future in jeopardy following the expulsion of Carnot, the three remaining musicians turned to an American talent to revive their fortunes.
Josiah “Big Willie” Gibbs was an American soloist who started his own hot chick research program in the most unlikely city of New Haven. While his tour with Heat Engine started with promise, he and Helmholtz eventually developed irreconcilable differences. Helmholtz, a dietician, performed with constant volume, while the American insisted on singing with pressure that didn’t change.
This was the end of the band, with Hermann and Rudolf retiring to Germany to spend time with family. Emile, devastated by the split, spent the rest of his days endlessly repeating calculations for ideal gasses, using a blowup doll and a thermal probe. Big Willie returned to America.
While Big Willie is the namesake of Gibbs Free Energy, the concept was actually invented by his long-suffering sister.
Big Willie was a life-long bachelor, and used the family home as a party pad even while his sister and her husband tried to raise a family there. Distraught by Gibbs’ exploits, his sister Julia derived the formula that bears Big Willie’s name with the express purpose of minimizing what she constantly referred to as “Gibbs’ Free Energy”. She naively gave Big Willie the broadcast rights to the formula, hoping that it would perhaps give her some peace and quiet around the house.
What Julia didn’t realize was this:
Although she, as a stable married woman, wished to minimize G, BigWillie was leading a lifestyle as energetic and unstable as possible. The main way he did this was to invent amphetamines, so that he could turn his concerts into ecstasy raves. By covering Clausius’s failed album with a new, psychedelic sound, Gibbs took Q=TdS to a whole new level, as his pharmacological supplements increased both the body temperature and the disorder of the girls who partook.
Julia originally defined Gibbs Free Energy as follows:
G=TdS-PdV
Gibbs merely substituted dQ for TdS, and work for PdV, to live the following lifestyle:
G = heat – work
For a young, unstable bachelor like Big Willie, the method of maximizing G was simple. Maximize heat, and minimize work. In plain English, get the hottest chick for the least effort. Young hotheads have followed in his steps ever since. So when you see a whispy-cheeked studmuffin taking his gorgeous hottie for granted, he isn’t being misogynist, or cruel; he’s just using thermodynamics. By maximizing his chemical potential, he hopes that his reactivity will be enhanced.
The definition, quantification, and calculation of heat was one of the most enduring and important scientific discoveries of the 19th century. For those unfamiliar with it, I will provide a brief historical synopsis of the quantification of hot chicks, and the development of the theory with which this value, Q, can be calculated. Finally, by using Gibbs' free energy, I will explain why young males are so insistent on maximizing the Q of their girlfriends.
The first attempts to rigorously define female attractiveness were done by the well known, and heavily idolized, Heat Engine. Heat Engine was a 19th century boy band with the following members:
- Rudolf Clausius: bass
- Emile Clapeyron: percussion
- Sadi Carnot: vocals
- Hermann Helmholtz: calorimeter
Like most boy band members, these four engineers possessed limited musical skill, and vast teenage lust. However, because lip-synching had not been invented in the 19th century, the boyz had to find a different mechanism with which to reconcile their talent with their testosterone. This is why they invented thermodynamics.
The original thermodynamic equations and models were invented to explain the inefficiencies inherent in translating musical prowess into sexual activity. In his seminal
Although that hit single was enough to immortalize Heat Engine, Rudolf Clausius one-upped his lead vocalist by composing the
dQ=dW+dE
In otherwords, the change in heat equals the work done plus the change in energy. This allowed mathematical confirmation of the empirical observation that hardworking, energetic chicks were hot. This simple, wholesome German formulation attracted little notice. However, his followup formulation, dQ=TdS, was considered obscene, and fell off the charts in three weeks.
Clausius and Emile Clapeyron went on to produce many successful duets on numerous seductive properties. These included the platinum selling “Second law of thermodynamics”, the irreversibly splendid “Entropy”, and an equation, named after themselves, that described the energy spent and entropy gained in changing phase or shedding clothes. They then tackled the thermodynamic concept that broke up the band, adiabatic cooling.
Carnot insisted that when a body expanded, she became less hot, a process that he described as adiabatic cooling. While the other three were in a jam session working out the instrumentals for this theory, he leaked it to the public, thus creating the multi-million dollar fashion, diet, and beauty industries, all based on the principle that denser is hotter.
Helmholtz and Clausius were both married to stout German hausfrauen by this time. When they found out about Carnot’s betrayal, they got all loga-rhythmic on his ass. In a blistering rush of calculus and musical genius, they proved that despite the change in temperature, adiabats not only conserved heat, but were also isentropic, and therefore reversible. Alas, the same did not hold for Carnot’s career, and he was banished from the band, dying from an STD on the streets of Paris later that year.
Unfortunately, by that time the myth of adiabatic cooling was firmly entrenched in the public imagination, and even a lurid refutation was not enough to change public opinion. The German prediction of heat conservation was confirmed near the end of the century in a shocking thermodynamic experiment. A deranged Italian highway engineer tried to increase the heat of half a dozen schoolgirls, by attempting to adiabatically compress them. Unfortunately, he used a steamroller to do so, which resulted in an irreversible, entropy-gaining densification to cardboard thickness. However, in the enquiry that followed, it was shown that the highly compressed corpses were in fact no hotter that the original schoolgirls had been before the attack. Sadly, the thermodynamic implication of this case was not one of the angles most heavily featured in the tabloids, so the myth survived unscathed.
Back in the 19th century, with Heat Engine’s future in jeopardy following the expulsion of Carnot, the three remaining musicians turned to an American talent to revive their fortunes.
Josiah “Big Willie” Gibbs was an American soloist who started his own hot chick research program in the most unlikely city of New Haven. While his tour with Heat Engine started with promise, he and Helmholtz eventually developed irreconcilable differences. Helmholtz, a dietician, performed with constant volume, while the American insisted on singing with pressure that didn’t change.
This was the end of the band, with Hermann and Rudolf retiring to Germany to spend time with family. Emile, devastated by the split, spent the rest of his days endlessly repeating calculations for ideal gasses, using a blowup doll and a thermal probe. Big Willie returned to America.
While Big Willie is the namesake of Gibbs Free Energy, the concept was actually invented by his long-suffering sister.
Big Willie was a life-long bachelor, and used the family home as a party pad even while his sister and her husband tried to raise a family there. Distraught by Gibbs’ exploits, his sister Julia derived the formula that bears Big Willie’s name with the express purpose of minimizing what she constantly referred to as “Gibbs’ Free Energy”. She naively gave Big Willie the broadcast rights to the formula, hoping that it would perhaps give her some peace and quiet around the house.
What Julia didn’t realize was this:
Although she, as a stable married woman, wished to minimize G, BigWillie was leading a lifestyle as energetic and unstable as possible. The main way he did this was to invent amphetamines, so that he could turn his concerts into ecstasy raves. By covering Clausius’s failed album with a new, psychedelic sound, Gibbs took Q=TdS to a whole new level, as his pharmacological supplements increased both the body temperature and the disorder of the girls who partook.
Julia originally defined Gibbs Free Energy as follows:
G=TdS-PdV
Gibbs merely substituted dQ for TdS, and work for PdV, to live the following lifestyle:
G = heat – work
For a young, unstable bachelor like Big Willie, the method of maximizing G was simple. Maximize heat, and minimize work. In plain English, get the hottest chick for the least effort. Young hotheads have followed in his steps ever since. So when you see a whispy-cheeked studmuffin taking his gorgeous hottie for granted, he isn’t being misogynist, or cruel; he’s just using thermodynamics. By maximizing his chemical potential, he hopes that his reactivity will be enhanced.
Saturday, December 16, 2006
Talking about the weather
Sciencewoman has been posting about the autumn rains in the Pacific Northwest recently, so for those people who are sick of rain, I have a somewhat different perspective: The Australian spring.
Below is a rainfall map for Aug-Oct of this year:

A map of the deviation from median rainfall is below:

(via the Bureau of Meteorology)
Canberra is the little blob in southern NSW, on the lower edge of the second largest red area. Obviously, this diagram does not say everything. After all, it’s a big continent, and the various regions have vastly different mean rainfalls and standard deviations from the mean. Additionally, many areas of Australia get little or no rainfall in some years, so it only takes a single storm to give them above average precipitation.
Never-the-less, it has been rather dry. The wheat harvest is looking to be 30-40% below average, the Murray River is in danger of drying up, and the Victorian bushfire alone have thus far consumed 550,000 hectares (860 square miles). So if anyone out there has any precipitation excess to their requirements, send it on over.
Below is a rainfall map for Aug-Oct of this year:

A map of the deviation from median rainfall is below:

(via the Bureau of Meteorology)
Canberra is the little blob in southern NSW, on the lower edge of the second largest red area. Obviously, this diagram does not say everything. After all, it’s a big continent, and the various regions have vastly different mean rainfalls and standard deviations from the mean. Additionally, many areas of Australia get little or no rainfall in some years, so it only takes a single storm to give them above average precipitation.
Never-the-less, it has been rather dry. The wheat harvest is looking to be 30-40% below average, the Murray River is in danger of drying up, and the Victorian bushfire alone have thus far consumed 550,000 hectares (860 square miles). So if anyone out there has any precipitation excess to their requirements, send it on over.
Friday, December 15, 2006
Pecan Pie
The art of pecan piery is a wild intersection of practice and passion, of ideology and indulgence. And yet, as with many of life’s fundamental pleasures, the basic principles are relatively straightforward.
For example, pecans are pecans. They are not, however, PEE-cans. A PEE-can is what, in Australia, we call a dunny. The most tasty and cultivated of the hickories, the pecan is a core ingredient, the presence of which defines this particular dessert. As a result, the only question when dealing with pecans is clast-supported vs. matrix supported. But I will leave that argument to diamictite and periglacial specialists.
A bit more variation is found in the caramelized substrate in which cements the pecans together. Here they are two independent binary choices, which yield a 2x2 matrix of sugary possibility. The first and defining choice is syrup: dark or light. The second choice is sugar: brown or white. Of the four possibilities generated, only two are widely used.
The light/white combination may only be consumed by pasty-lipped, precious Yankees, and has no place in the pantheon of pecan pie. If you want something that bland, move to Sweden, or replace the pecans with cashews. Or macadamias.
At the other extreme is the dark syrup, brown sugar pie. I’m sure that deep in the steamy bayous of the Mississippi Delta, there exists a testosterone-fuelled, hairy-chested John Henry of the pastry-rolling industry, who might just be man enough to bake such a dessert. But short of augmentation by an East German swimming doctor, my masculinity is nowhere near the level required to attempt such a pie.
This leads two possibilities: light syrup with brown sugar, and dark syrup with white sugar. My down-home, good ol’ boy and girl Southern friends and relatives tell me that the dark syrup is the more authentic of the two. But I prefer the light syrup for two reasons.
The first reason I prefer the light syrup is a matter of upbringing. My parents, who possess the professional drive and career development acumen that has thus far eluded me, chose my fate when I was born. Hoping I would grow up to be a classical hero, they bundled me up as a baby, put me in a chest, and cast it into the James River, hoping that it would be found by a fisherman with the capabilities of raising orphans into monster-slaying demigods.
The chest floated up the Chesapeake Bay, through the intracoastal waterway, and across the Delaware River, washing ashore on the Jersey shore, just north of the Mason-Dixon line. True to form, once ashore I was adopted by the wolves, mallrats, and members of the Gotti family that call New Jersey home.
As a result of this peculiar upbringing, I am not a real southerner. My palate sometimes reflects this high latitude upbringing, as my tastebuds have been ruined by overexposure to hoagies and bagels.
The second reason that I prefer light syrup is that dark Karo is hard to find in Australia. The light stuff is more widely available, and can be replaced with non-corn glucose syrup sourced from domestic sources. With Karo going for over $6 a bottle, this is not a bad thing.
My current recipe is a blend of book-pie, internet heresy, and old-time tradition passed down through several families not my own. Experimentation has warped the recipe beyond recognition, so I present the current version herewith.
Composite pecan pie
3/4 cup white corn syrup
Tablespoon maple syrup
Tablespoon honey
1/2 cup brown sugar
3 eggs, lightly beaten
2 cups quartered pecans
Handful of unbroken pecans
4 tablespoons butter
1 teaspoon vanilla
1/4 teaspoon each
ground cloves and allspice
1/2 teaspoon cinnamon
Boil sugar, honey, and syrups together for 3 minutes. Blend eggs with fork (not beater). Melt butter in syrup, and stir together over low heat for 30 seconds. Pour egg into syrup, stirring vigorously so the egg does not cook. Add vanilla, salt, spices and broken pecans. Pour into raw pie crust. Cover with surface layer of whole pecans. Bake at 180C for 45 minutes, or until steel skewer comes out gooless.
For example, pecans are pecans. They are not, however, PEE-cans. A PEE-can is what, in Australia, we call a dunny. The most tasty and cultivated of the hickories, the pecan is a core ingredient, the presence of which defines this particular dessert. As a result, the only question when dealing with pecans is clast-supported vs. matrix supported. But I will leave that argument to diamictite and periglacial specialists.
A bit more variation is found in the caramelized substrate in which cements the pecans together. Here they are two independent binary choices, which yield a 2x2 matrix of sugary possibility. The first and defining choice is syrup: dark or light. The second choice is sugar: brown or white. Of the four possibilities generated, only two are widely used.
The light/white combination may only be consumed by pasty-lipped, precious Yankees, and has no place in the pantheon of pecan pie. If you want something that bland, move to Sweden, or replace the pecans with cashews. Or macadamias.
At the other extreme is the dark syrup, brown sugar pie. I’m sure that deep in the steamy bayous of the Mississippi Delta, there exists a testosterone-fuelled, hairy-chested John Henry of the pastry-rolling industry, who might just be man enough to bake such a dessert. But short of augmentation by an East German swimming doctor, my masculinity is nowhere near the level required to attempt such a pie.
This leads two possibilities: light syrup with brown sugar, and dark syrup with white sugar. My down-home, good ol’ boy and girl Southern friends and relatives tell me that the dark syrup is the more authentic of the two. But I prefer the light syrup for two reasons.
The first reason I prefer the light syrup is a matter of upbringing. My parents, who possess the professional drive and career development acumen that has thus far eluded me, chose my fate when I was born. Hoping I would grow up to be a classical hero, they bundled me up as a baby, put me in a chest, and cast it into the James River, hoping that it would be found by a fisherman with the capabilities of raising orphans into monster-slaying demigods.
The chest floated up the Chesapeake Bay, through the intracoastal waterway, and across the Delaware River, washing ashore on the Jersey shore, just north of the Mason-Dixon line. True to form, once ashore I was adopted by the wolves, mallrats, and members of the Gotti family that call New Jersey home.
As a result of this peculiar upbringing, I am not a real southerner. My palate sometimes reflects this high latitude upbringing, as my tastebuds have been ruined by overexposure to hoagies and bagels.
The second reason that I prefer light syrup is that dark Karo is hard to find in Australia. The light stuff is more widely available, and can be replaced with non-corn glucose syrup sourced from domestic sources. With Karo going for over $6 a bottle, this is not a bad thing.
My current recipe is a blend of book-pie, internet heresy, and old-time tradition passed down through several families not my own. Experimentation has warped the recipe beyond recognition, so I present the current version herewith.
Composite pecan pie
3/4 cup white corn syrup
Tablespoon maple syrup
Tablespoon honey
1/2 cup brown sugar
3 eggs, lightly beaten
2 cups quartered pecans
Handful of unbroken pecans
4 tablespoons butter
1 teaspoon vanilla
1/4 teaspoon each
ground cloves and allspice
1/2 teaspoon cinnamon
Boil sugar, honey, and syrups together for 3 minutes. Blend eggs with fork (not beater). Melt butter in syrup, and stir together over low heat for 30 seconds. Pour egg into syrup, stirring vigorously so the egg does not cook. Add vanilla, salt, spices and broken pecans. Pour into raw pie crust. Cover with surface layer of whole pecans. Bake at 180C for 45 minutes, or until steel skewer comes out gooless.
Monday, December 11, 2006
Neandertal ≠ Chauvinist
In their recent Current Anthropology article, Khun and Steiner suggest that one of the important behavioral differences between Neandertal and modern humans was the development of gender-based division of labour. While many of the implications and predictions of this theory are discussed in the comments and reply section, there is an important corollary which the research and debate neglected to mention:
Neandertals are not sexist.
This is an important point. In contemporary western society the (stereo)typical liberal feminist spokeswoman will often place herself at odds with those of us who are block-headed, flesh-eating primitives with a genome that is 50,000 years out of date.
Ladies Females, we are not the enemy. We possess neither the cultural sophistication nor the hierarchical mindset necessary to associate roles and gender. As far as we are concerned, anyone- man, woman, or child- can make a living spearing buffalo, working in a sweatshop, or building mass spectrometers. So cut us some slack.
The bigots you want to look out for are the hairless, gracile, anatomically modern boys; the ones with complexity and sophistication. Those males will gladly pigeonhole you into roles such as gatherer, secretary, or permanent adjunct. It is the natural consequence of their compartmentalized worldview, which includes such trivialities as artistic expression, a varied, vegetable-rich diet, and projectile weaponry. But you don’t have to put up with it; we Neandertals are far more egalitarian. As long as you don’t mind killing megafauna for a living, y’all are welcome to join ourhunting party department on equal and unbiased terms.
One final point: Please cease comparing offensively primitive males to cavemen. Caveman is an imprecise term, as caves have been used for shelter throughout hominid evolution. Specifically, in the European context, it can refer to either a dinky-di, non-judgmental Neandertal, or a cave-painting, patriarchal Cro-magnon. We prefer not to be associated with such avant-garde Frenchmen.
Neandertals are not sexist.
This is an important point. In contemporary western society the (stereo)typical liberal feminist spokeswoman will often place herself at odds with those of us who are block-headed, flesh-eating primitives with a genome that is 50,000 years out of date.
The bigots you want to look out for are the hairless, gracile, anatomically modern boys; the ones with complexity and sophistication. Those males will gladly pigeonhole you into roles such as gatherer, secretary, or permanent adjunct. It is the natural consequence of their compartmentalized worldview, which includes such trivialities as artistic expression, a varied, vegetable-rich diet, and projectile weaponry. But you don’t have to put up with it; we Neandertals are far more egalitarian. As long as you don’t mind killing megafauna for a living, y’all are welcome to join our
One final point: Please cease comparing offensively primitive males to cavemen. Caveman is an imprecise term, as caves have been used for shelter throughout hominid evolution. Specifically, in the European context, it can refer to either a dinky-di, non-judgmental Neandertal, or a cave-painting, patriarchal Cro-magnon. We prefer not to be associated with such avant-garde Frenchmen.
Friday, December 08, 2006
Sunday, December 03, 2006
Shrill smokescreens and radioactive bananas
In a dashing blaze of opportunistic fear-mongering, the New York Times op-ed page is reporting that cigarettes, in addition to their usually toxins, also contain radioactive polonium-210. This is the same isotope used to assassinate Victor Litvinenko. The article, long on analogy and short on math, even goes so far to suggest that the total polonium dosage of second hand smoke in London could equal that which killed the former Russian spy. So, how much radiation is 0.04 picocuries?
Why, 1.48x10-3 decays per second, of course. That’s about one decay every ten minutes. You’d have to be in a very deep, shielded room to detect that sort of signal above the cosmic ray background, and if your shielded room was made of cement, sandstone, or granite, the decays from naturally occurring radioactive minerals would also dwarf your polonium signal.
For analogy lovers, here’s a more correct one that what Professor Proctor has dished out: Potassium, which is a vital nutrient, has a slightly radioactive minor isotope, 40K. With an isotopic abundance of .01% and a half-life of 1.25 billion years, a banana with 450 mg of K will kick out 14 decays every second. So a banana is over nine thousand times more radioactive than the polonium in a cigarette.
Now, how many cigarettes would it take to get a lethal dose? Well, the LD 50 for ingestion is around 8 million becquerels (decays/sec). So with 1.48x10-3 Bq per fag, you would need about 5.4 billion of them to accumulate a lethal dose of polonium. I reckon the nicotine would get you first.
Professor Proctor writes, “London’s smokers (and those Londoners exposed to secondhand smoke), taken as a group, probably inhale more polonium 210 on any given day than the former spy ingested with his sushi.” Can this be true? Well, with a lethal dose 5.4 billion times greater than that of a fag, and assuming that 5.4 million Londoners smoke, they’d have to suck down a thousand cigs a day (50 packs) in order for the figures to be correct. Muscovites may think a 50 pack day is cold turkey, but Londoners? I doubt it.
Professor Proctor obviously thinks that the risk of smoking justifies incorrect arithmetic and easily refutable generalizations. Hopefully, my calculations will allow all my smoking readers to rest easy tonight, secure in the knowledge that it will be the tar and the nicotine that kills them, not the 210Po.
p.s. As a geologist, I usually work in years, not seconds, so the first time I did the banana calculation, I instinctively calculated decays per year, and assumed I had seconds. However, I quickly decided that if 17 billion Bq was the dose from a typical banana, then I had bigger things to worry about than this blog.
Why, 1.48x10-3 decays per second, of course. That’s about one decay every ten minutes. You’d have to be in a very deep, shielded room to detect that sort of signal above the cosmic ray background, and if your shielded room was made of cement, sandstone, or granite, the decays from naturally occurring radioactive minerals would also dwarf your polonium signal.
For analogy lovers, here’s a more correct one that what Professor Proctor has dished out: Potassium, which is a vital nutrient, has a slightly radioactive minor isotope, 40K. With an isotopic abundance of .01% and a half-life of 1.25 billion years, a banana with 450 mg of K will kick out 14 decays every second. So a banana is over nine thousand times more radioactive than the polonium in a cigarette.
Now, how many cigarettes would it take to get a lethal dose? Well, the LD 50 for ingestion is around 8 million becquerels (decays/sec). So with 1.48x10-3 Bq per fag, you would need about 5.4 billion of them to accumulate a lethal dose of polonium. I reckon the nicotine would get you first.
Professor Proctor writes, “London’s smokers (and those Londoners exposed to secondhand smoke), taken as a group, probably inhale more polonium 210 on any given day than the former spy ingested with his sushi.” Can this be true? Well, with a lethal dose 5.4 billion times greater than that of a fag, and assuming that 5.4 million Londoners smoke, they’d have to suck down a thousand cigs a day (50 packs) in order for the figures to be correct. Muscovites may think a 50 pack day is cold turkey, but Londoners? I doubt it.
Professor Proctor obviously thinks that the risk of smoking justifies incorrect arithmetic and easily refutable generalizations. Hopefully, my calculations will allow all my smoking readers to rest easy tonight, secure in the knowledge that it will be the tar and the nicotine that kills them, not the 210Po.
p.s. As a geologist, I usually work in years, not seconds, so the first time I did the banana calculation, I instinctively calculated decays per year, and assumed I had seconds. However, I quickly decided that if 17 billion Bq was the dose from a typical banana, then I had bigger things to worry about than this blog.
Cricket
Curse those wiley English, and their ability to learn from, adapt to, and plan around our neolithic style of play.
Monday, November 27, 2006
A match made in heaven
I read a couple of interesting articles on the web last week, but didn’t see how they relate to each other until just now.
The first was an article by the New York Times, describing how Mythbusters is the best science show on television. I happen to agree with this sentiment. If fact, I know of no other TV show that demonstrates how to test and refine a hypothesis as well.
The second article was a blog at Arms Control Wonk about how a nuclear weapon could be built from surplus military equipment (and a whole pile of Uranium). What really caught my notice here was the number of people who commented on whether the method described would work, and how it could be improved.
Now, I don’t entirely understand the temptation to post recipes for nuclear weapons on the internet- I’ve personally never uploaded instructions for creating anything more dangerous than a chocolate chip cookie. But my scientifically trained eye did notice that this wide variety of opinions and unsubstantiated prognostication did indicate a glaring shortfall: The lack of experimental data. And that’s where Mythbusters comes in.
Adam and Jamie are the masters of refuting bombastic urban legends through experimentation. So instead of polluting the internet with untested ideas, perhaps we should just give the Mythbusters the following hypothesis:
It would be a win-win episode. If the myth us busted, then we could all breathe easier at night, knowing that nuclear terrorism is more difficult than it appears.
If the myth is partially confirmed- say, by a North Korean-style fizzle that irradiates all of Petaluma, then we can emphasize the unpredictability and danger of playing with critical masses of fissile material.
And finally, if the myth is confirmed by vaporizing the entire San Francisco metropolitan area, then at least we have the sort of visual spectacle that is appropriate for a season finale. Such a prospect might even appeal to the ultra-conservative Republican base, a community that could use a bit of extra scientific education.
If it's a hit, we could even follow it up with a test of some similar myths. For example:
Myth: Anthrax can be passed through the mail.
Myth: Most of today's population has lost resistance to small pox.
and finally
Myth: A large scale nuclear war will offset global warming with nuclear winter.
The first was an article by the New York Times, describing how Mythbusters is the best science show on television. I happen to agree with this sentiment. If fact, I know of no other TV show that demonstrates how to test and refine a hypothesis as well.
The second article was a blog at Arms Control Wonk about how a nuclear weapon could be built from surplus military equipment (and a whole pile of Uranium). What really caught my notice here was the number of people who commented on whether the method described would work, and how it could be improved.
Now, I don’t entirely understand the temptation to post recipes for nuclear weapons on the internet- I’ve personally never uploaded instructions for creating anything more dangerous than a chocolate chip cookie. But my scientifically trained eye did notice that this wide variety of opinions and unsubstantiated prognostication did indicate a glaring shortfall: The lack of experimental data. And that’s where Mythbusters comes in.
Adam and Jamie are the masters of refuting bombastic urban legends through experimentation. So instead of polluting the internet with untested ideas, perhaps we should just give the Mythbusters the following hypothesis:
Myth: Shooting two bits of highly enriched uranium together will cause a nuclear explosion
It would be a win-win episode. If the myth us busted, then we could all breathe easier at night, knowing that nuclear terrorism is more difficult than it appears.
If the myth is partially confirmed- say, by a North Korean-style fizzle that irradiates all of Petaluma, then we can emphasize the unpredictability and danger of playing with critical masses of fissile material.
And finally, if the myth is confirmed by vaporizing the entire San Francisco metropolitan area, then at least we have the sort of visual spectacle that is appropriate for a season finale. Such a prospect might even appeal to the ultra-conservative Republican base, a community that could use a bit of extra scientific education.
If it's a hit, we could even follow it up with a test of some similar myths. For example:
Myth: Anthrax can be passed through the mail.
Myth: Most of today's population has lost resistance to small pox.
and finally
Myth: A large scale nuclear war will offset global warming with nuclear winter.
Sunday, November 26, 2006
210Po
Last week, the former Russian spymaster Alexander Litvinenko was killed by radiation poisoning after ingesting a dose of 210Po. I am not qualified to comment on the political ramifications of this radiological attack, I can give the bare-bones information on 210Po.
There are three basic ways to make short-lived radionuclides here on Earth. The first is to collect the short-lived intermediate decay products of Th or U isotopes, which undergo complex decay chains from the relatively stable initial isotopes, through increasingly unstable decay products, to Pb, which is stable. The second is to fission a heavy nuclide into unstable daughter products. The third is to irradiate a stable or long-lived nuclide such as 39K, 238U or 59Co in a reactor or particle accelerator to produce a less stable daughter, like 39Ar, 239Pu, or 60Co.
210Po -> 206Pb is the final decay in the 238U -> 206Pb decay system. As such, it is present in natural uranium ore in concentrations that are inversely proportional to the ratio of the decay constants of 210Po and 238U . This ratio is about 8.6x10-11. So for every kg of U in natural ore, there is 86ng of 210Po. (alternatively, that is 86ug/ton).
There have been reports that the soviets stockpiled 210Po during the cold war. While this may be true, it is unlikely that this is the source for the poison used today. 210Po has a half life of 138 days, and the cold war ended about 5800 days, or 14 210Po half-lives, ago. Thus essentially all of a cold war stockpile would have disappeared by now.
There are several options for 210Po generators, including 210Pb (half-life 22 years), and 226Ra (half-life 1600 years). In fact, polonium was originally discovered by Marie & Pierre Curie, after their isolation of Radium.
210Po can also be formed by neutron irradiation of 209Bi, at least according to Wikipedia. It is not abundant in spent nuclear fuel.
Anyway, the point of all this is that whoever killed the guy must have had access to either a reactor, a supply of the highly radioactive isotopes 226Ra or 210Pb, or an industrial scale U processing facility and a chemistry lab that can deal with serious amounts of radiation. It is way more sophisticated than stealing a spent fuel rod or a medical radiation source, and thus is considerably more worrisome than a mere dirty bomb. This radiological attack was obviously perpetrated by someone who knew what they were doing, and had access to some serious infrastructure.
There are three basic ways to make short-lived radionuclides here on Earth. The first is to collect the short-lived intermediate decay products of Th or U isotopes, which undergo complex decay chains from the relatively stable initial isotopes, through increasingly unstable decay products, to Pb, which is stable. The second is to fission a heavy nuclide into unstable daughter products. The third is to irradiate a stable or long-lived nuclide such as 39K, 238U or 59Co in a reactor or particle accelerator to produce a less stable daughter, like 39Ar, 239Pu, or 60Co.
210Po -> 206Pb is the final decay in the 238U -> 206Pb decay system. As such, it is present in natural uranium ore in concentrations that are inversely proportional to the ratio of the decay constants of 210Po and 238U . This ratio is about 8.6x10-11. So for every kg of U in natural ore, there is 86ng of 210Po. (alternatively, that is 86ug/ton).
There have been reports that the soviets stockpiled 210Po during the cold war. While this may be true, it is unlikely that this is the source for the poison used today. 210Po has a half life of 138 days, and the cold war ended about 5800 days, or 14 210Po half-lives, ago. Thus essentially all of a cold war stockpile would have disappeared by now.
There are several options for 210Po generators, including 210Pb (half-life 22 years), and 226Ra (half-life 1600 years). In fact, polonium was originally discovered by Marie & Pierre Curie, after their isolation of Radium.
210Po can also be formed by neutron irradiation of 209Bi, at least according to Wikipedia. It is not abundant in spent nuclear fuel.
Anyway, the point of all this is that whoever killed the guy must have had access to either a reactor, a supply of the highly radioactive isotopes 226Ra or 210Pb, or an industrial scale U processing facility and a chemistry lab that can deal with serious amounts of radiation. It is way more sophisticated than stealing a spent fuel rod or a medical radiation source, and thus is considerably more worrisome than a mere dirty bomb. This radiological attack was obviously perpetrated by someone who knew what they were doing, and had access to some serious infrastructure.
Friday, November 24, 2006
Saturday, November 18, 2006
Phase equilibria of pie crust
With American Thanksgiving and Christmas rapidly approaching, the pie baking season is rapidly approaching. One of the most important, but least quantified, aspects of pie creation is the crustal composition. A simple ternary phase diagram for three-phase pie crust is presented below.
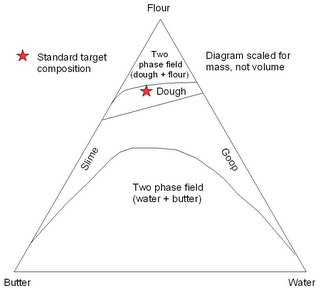
While the “traditional composition” point is plotted to scale, the positions and shapes of the curve are poorly constrained approximations. Lack of accurate thermodynamic data for the system precludes accurate prediction of these fields. It is the shapes and positions of the top two curves that is of paramount importance; anyone who reaches the butter-water two phase field should be banished from the kitchen.
As anyone with baking experience knows, the stability region for pie crust is a relatively small area on the wet side of the two phase flour + dough field. This field is generally approached by adding water to a flour/butter mixture, as is shown below.

However, if the approximated slopes shown above are correct, then a radical new approach to crustal formation might be advisable. By generating a flour-water mixture, and then adding butter, a wider range of valid crustal compositions should be achievable before exiting the edible portion of this phase diagram. This approach is shown below.

I will be baking at least three pies this week for T-day. I might have a go at this radical new approach, to see if it yields additional information on the slope of the upper two phase boundaries. I suspect they might be steeper than the lines shown here. Assuming I can actually get a cheap, accurate kitchen scale, there may be more reports on the thermopienamics of this system sometime next week.

While the “traditional composition” point is plotted to scale, the positions and shapes of the curve are poorly constrained approximations. Lack of accurate thermodynamic data for the system precludes accurate prediction of these fields. It is the shapes and positions of the top two curves that is of paramount importance; anyone who reaches the butter-water two phase field should be banished from the kitchen.
As anyone with baking experience knows, the stability region for pie crust is a relatively small area on the wet side of the two phase flour + dough field. This field is generally approached by adding water to a flour/butter mixture, as is shown below.

However, if the approximated slopes shown above are correct, then a radical new approach to crustal formation might be advisable. By generating a flour-water mixture, and then adding butter, a wider range of valid crustal compositions should be achievable before exiting the edible portion of this phase diagram. This approach is shown below.

I will be baking at least three pies this week for T-day. I might have a go at this radical new approach, to see if it yields additional information on the slope of the upper two phase boundaries. I suspect they might be steeper than the lines shown here. Assuming I can actually get a cheap, accurate kitchen scale, there may be more reports on the thermopienamics of this system sometime next week.
Thursday, November 16, 2006
Kuril Islands earthquake and tsunami
Earthquake info:
http://earthquake.usgs.gov/eqcenter/eqinthenews/2006/usvcam/
Tsunami info (the tsunami wanring and watch has been canceled):
http://wcatwc.arh.noaa.gov/eventmap.html
http://earthquake.usgs.gov/eqcenter/eqinthenews/2006/usvcam/
Tsunami info (the tsunami wanring and watch has been canceled):
http://wcatwc.arh.noaa.gov/eventmap.html
Wednesday, November 15, 2006
Eye of Newt and blur of science
Double double toil and trouble.
Fire burn and cauldron bubble.
One of the problems facing scientists and science promoters is that the human brain has not evolved to naturally gravitate towards clever experiments or robust theorems. As a result, journalists and educators often resort to using a variety of non-scientific techniques to catch the public’s interest or to explain the significance of a particular discovery. The problem with this approach is that it blurs the borders between science and the non-scientific tools used for education. It is difficult to complain about liturgists, fraudsters, or entertainers intruding into the arena of science when their traditional techniques are used for science promotion.
I suspect that entire journals could be filled with theories, explanations, or apologies for various aspects of and solutions to this problem, but being an illiterate lab techo, I haven’t read any of them. So I will ignorantly suggest a simple way of judging the usefulness of any particular unscientific selling technique: the cost-benefit analysis. If a particular method has limited explanatory power, and has a high chance of causing confusion or misunderstanding, then it should be avoided. One such high cost, low benefit literary device is nomenclatural overload, and its poor cousin, technobabble.
Modern science is complicated. But despite that complication, the particular experiments and observations that are undertaken are generally done for specific, definable reasons. Simply dumping the names or generalizations about a field onto the page without explaining them merely creates the illusion that science is a collection of arcane trivia, and not the amalgamation of knowledge based on prediction and observation. The practice of name dropping and information overloading has the effect of reducing science to alchemy, where arcane ingredients are combined and channeled without any overarching principles. This is obviously a risky approach:
Adder’s fork, and blind worm’s sting,
Lizard’s leg, and howlet’s wing-
For a charm of pow’rful trouble,
Like a hell-broth boil and bubble.
The solution is fairly obvious- if you’re writing an article, explain why things are important before you discuss what the result of measuring them was. An example of how this should, but doesn’t happen can be found in this week’s New York Times science section, in the article, "Ancient Crash, Epic Wave."
The offending excerpt:
“When a chondritic meteor, the most common kind, vaporizes upon impact in the ocean, those three metals [iron, nickel and chrome] are formed in the same relative proportions as seen in the microfossils, Dr. Abbott said.”
The reason behind these analyses is fairly simple, so it is a shame that this otherwise excellent article didn’t bother explaining it. For any non-geologists who have wandered into this site by accident, here is the story:
When the planets and asteroids originally formed early in the solar system’s history, the larger ones heated up and melted, and the immiscible liquid metal and molten rock differentiated to form a metallic core and a silicate mantle. Small asteroids did not generate enough heat to melt, and remained undifferentiated. These are the chondrites, and they contain a mix of metal and silicate that condensed from the primordial solar nebula.
Because the Earth is differentiated, most of the elements that dissolve into metal instead of magma are contained in the core, and thus are relatively rare at the surface. These elements, called “siderophiles” (iron lovers) are thus more common in undifferentiated bodies than they are in the Earth’s crust. So when a chondrite hits the earth, the ejecta is enriched in siderophiles such as Fe, Cr, and Ni. Iridium is also a siderophile, so the search for nickel and chromium is based on the same principle as the search for the Ir anomaly at the K/T boundary, which marked the end of the Mesozoic (the age of dinosaurs).
Obviously, these elements are not “formed” in the impact. They are just particles from the impactor, which can be identified because the impactor and the silicate Earth have very different siderophile concentrations.
Of course, getting scared off by terminology and avoiding it is just as problematic as simply namedropping undefined terms. But in science, things often have names for a reason, so explaining what that reason is can go a long way towards illuminating the subject at hand.
Fire burn and cauldron bubble.
One of the problems facing scientists and science promoters is that the human brain has not evolved to naturally gravitate towards clever experiments or robust theorems. As a result, journalists and educators often resort to using a variety of non-scientific techniques to catch the public’s interest or to explain the significance of a particular discovery. The problem with this approach is that it blurs the borders between science and the non-scientific tools used for education. It is difficult to complain about liturgists, fraudsters, or entertainers intruding into the arena of science when their traditional techniques are used for science promotion.
I suspect that entire journals could be filled with theories, explanations, or apologies for various aspects of and solutions to this problem, but being an illiterate lab techo, I haven’t read any of them. So I will ignorantly suggest a simple way of judging the usefulness of any particular unscientific selling technique: the cost-benefit analysis. If a particular method has limited explanatory power, and has a high chance of causing confusion or misunderstanding, then it should be avoided. One such high cost, low benefit literary device is nomenclatural overload, and its poor cousin, technobabble.
Modern science is complicated. But despite that complication, the particular experiments and observations that are undertaken are generally done for specific, definable reasons. Simply dumping the names or generalizations about a field onto the page without explaining them merely creates the illusion that science is a collection of arcane trivia, and not the amalgamation of knowledge based on prediction and observation. The practice of name dropping and information overloading has the effect of reducing science to alchemy, where arcane ingredients are combined and channeled without any overarching principles. This is obviously a risky approach:
Adder’s fork, and blind worm’s sting,
Lizard’s leg, and howlet’s wing-
For a charm of pow’rful trouble,
Like a hell-broth boil and bubble.
The solution is fairly obvious- if you’re writing an article, explain why things are important before you discuss what the result of measuring them was. An example of how this should, but doesn’t happen can be found in this week’s New York Times science section, in the article, "Ancient Crash, Epic Wave."
The offending excerpt:
“When a chondritic meteor, the most common kind, vaporizes upon impact in the ocean, those three metals [iron, nickel and chrome] are formed in the same relative proportions as seen in the microfossils, Dr. Abbott said.”
The reason behind these analyses is fairly simple, so it is a shame that this otherwise excellent article didn’t bother explaining it. For any non-geologists who have wandered into this site by accident, here is the story:
When the planets and asteroids originally formed early in the solar system’s history, the larger ones heated up and melted, and the immiscible liquid metal and molten rock differentiated to form a metallic core and a silicate mantle. Small asteroids did not generate enough heat to melt, and remained undifferentiated. These are the chondrites, and they contain a mix of metal and silicate that condensed from the primordial solar nebula.
Because the Earth is differentiated, most of the elements that dissolve into metal instead of magma are contained in the core, and thus are relatively rare at the surface. These elements, called “siderophiles” (iron lovers) are thus more common in undifferentiated bodies than they are in the Earth’s crust. So when a chondrite hits the earth, the ejecta is enriched in siderophiles such as Fe, Cr, and Ni. Iridium is also a siderophile, so the search for nickel and chromium is based on the same principle as the search for the Ir anomaly at the K/T boundary, which marked the end of the Mesozoic (the age of dinosaurs).
Obviously, these elements are not “formed” in the impact. They are just particles from the impactor, which can be identified because the impactor and the silicate Earth have very different siderophile concentrations.
Of course, getting scared off by terminology and avoiding it is just as problematic as simply namedropping undefined terms. But in science, things often have names for a reason, so explaining what that reason is can go a long way towards illuminating the subject at hand.
Saturday, November 11, 2006
A bit of perspective, lab style
High School science is not a particularly memorable period in one’s journey through life. The science geeks generally know everything already, while the people who don’t really care are hardly inspired to learn. But my second year of high school physics had a few memorable labs.
Our school didn’t have the funds/ organization/ inclination to offer AP physics, so the “advanced” physics had one of the physics teachers dredging up all this crazy ancient equipment and teaching us the physics that they demonstrated. There were two labs, in particular, which I remember.
The first was digging up and fixing an old single grating diffractometer and looking at the discharges from various gas tubes to learn all about both diffraction and gas excitation. The culmination of this was figuring out what the fluoro tubes that lit the lab must be filled with- it was easy to look up, but cool to see that ordinary real life was made of the same elements, wavelengths, and concepts that were stored in the back of the physics cabinet.
The second lab I remember was radioactivity. The cabinet (perhaps set up by Dr. Calgari?) contained a half a dozen Geiger counters. First we learned safety procedures- which include Geiger-countering each other at the end of each lab to detect spills. Next, we looked at the radioactivity of various normal or natural items. Finally, the teacher brought out the radioactive generators. These were little disk-shaped things, about the size of a small stack of poker chips, through which a dilute acid was poured. The liquid that emerged was radioactive, and our task was to count it throughout the class to determine the halflife, from which we were to identify the isotope being extracted. I know I got the isotope wrong, and I still can’t remember what it was, exactly- the halflife was less than the class length, though, so my best guess- 15 years later- would be 223Fr.
Of course, we all had to Geiger-counter each other at the end, just to make sure nobody spilled anything, and this is where some clown snuck the pitchblende ore up behind the counter as it dropped past the beltline of a particularly nervous doctor wannabe. Being a fairly excitable sort of kid, he went on for weeks about how dangerous and stupid the entire lab was, how those extra handful of decays had needlessly put him at risk, and all the other prattlings of an angry, humiliated young man.
He sort of had a point- I mean, radiation is dangerous, and even though we were generally well-behaved and conscientious, I’m not really sure what would have happened if somebody had spilled a beaker on themselves. But the magnitude and importance of the risk was brought into clearer focus after Christmas that year.
When we came back from Christmas break, our physics teacher was gone, and the school’s other teacher took over the class for the rest of the year. We graduated in 1991, and our teacher, who was in the army reserve, had been called up to teach kids a year older than us how to drive tanks, in preparation for the liberation of Kuwait in the first gulf war.
No longer was he teaching us the physical processes illustrated by trace concentrations of U decay chain products. Instead, he was showing army recruits how to take care of, load, and fire shells made of depleted uranium.
It was then that we realized that all of the chemistry, physics, and other science that we were taught to use caution around was also used for the deliberate killing of other human beings. It is one thing to have a bottle of nitrates say, “warning: fire hazard.” It is another for someone to use the same basic decomposition reaction in a bomb or shell aimed at your head. Technology can do some nasty things to the human body, and one of my key high school revelations was that people my age would willingly put themselves in the way of technology’s most dangerous creations.
Needless to say, complaining about a high school lab seemed a little bit petty compared to the idea of going off to war. This realization was heightened when I turned in my selective service card at the post office on the President’s deadline for Iraqi troops to leave Kuwait. The bombing campaign started 2 days later.
Today is Remembrance Day. So spare a thought for all the service men and women who are putting themselves in harm’s way. They put themselves in the way of science’s most destructive and lethal applications, so that we don’t have to worry about anything more traumatic than traffic, or eye strain, or any of the other molehills that we tectonically uplift in the day to day existence of our overly civilized lives.
Our school didn’t have the funds/ organization/ inclination to offer AP physics, so the “advanced” physics had one of the physics teachers dredging up all this crazy ancient equipment and teaching us the physics that they demonstrated. There were two labs, in particular, which I remember.
The first was digging up and fixing an old single grating diffractometer and looking at the discharges from various gas tubes to learn all about both diffraction and gas excitation. The culmination of this was figuring out what the fluoro tubes that lit the lab must be filled with- it was easy to look up, but cool to see that ordinary real life was made of the same elements, wavelengths, and concepts that were stored in the back of the physics cabinet.
The second lab I remember was radioactivity. The cabinet (perhaps set up by Dr. Calgari?) contained a half a dozen Geiger counters. First we learned safety procedures- which include Geiger-countering each other at the end of each lab to detect spills. Next, we looked at the radioactivity of various normal or natural items. Finally, the teacher brought out the radioactive generators. These were little disk-shaped things, about the size of a small stack of poker chips, through which a dilute acid was poured. The liquid that emerged was radioactive, and our task was to count it throughout the class to determine the halflife, from which we were to identify the isotope being extracted. I know I got the isotope wrong, and I still can’t remember what it was, exactly- the halflife was less than the class length, though, so my best guess- 15 years later- would be 223Fr.
Of course, we all had to Geiger-counter each other at the end, just to make sure nobody spilled anything, and this is where some clown snuck the pitchblende ore up behind the counter as it dropped past the beltline of a particularly nervous doctor wannabe. Being a fairly excitable sort of kid, he went on for weeks about how dangerous and stupid the entire lab was, how those extra handful of decays had needlessly put him at risk, and all the other prattlings of an angry, humiliated young man.
He sort of had a point- I mean, radiation is dangerous, and even though we were generally well-behaved and conscientious, I’m not really sure what would have happened if somebody had spilled a beaker on themselves. But the magnitude and importance of the risk was brought into clearer focus after Christmas that year.
When we came back from Christmas break, our physics teacher was gone, and the school’s other teacher took over the class for the rest of the year. We graduated in 1991, and our teacher, who was in the army reserve, had been called up to teach kids a year older than us how to drive tanks, in preparation for the liberation of Kuwait in the first gulf war.
No longer was he teaching us the physical processes illustrated by trace concentrations of U decay chain products. Instead, he was showing army recruits how to take care of, load, and fire shells made of depleted uranium.
It was then that we realized that all of the chemistry, physics, and other science that we were taught to use caution around was also used for the deliberate killing of other human beings. It is one thing to have a bottle of nitrates say, “warning: fire hazard.” It is another for someone to use the same basic decomposition reaction in a bomb or shell aimed at your head. Technology can do some nasty things to the human body, and one of my key high school revelations was that people my age would willingly put themselves in the way of technology’s most dangerous creations.
Needless to say, complaining about a high school lab seemed a little bit petty compared to the idea of going off to war. This realization was heightened when I turned in my selective service card at the post office on the President’s deadline for Iraqi troops to leave Kuwait. The bombing campaign started 2 days later.
Today is Remembrance Day. So spare a thought for all the service men and women who are putting themselves in harm’s way. They put themselves in the way of science’s most destructive and lethal applications, so that we don’t have to worry about anything more traumatic than traffic, or eye strain, or any of the other molehills that we tectonically uplift in the day to day existence of our overly civilized lives.
Wednesday, November 08, 2006
Jan Veizen’s cosmic ray climatology
Last week we had Jan Veizen, a long-ago ANU alumnus, come back to give a talk about how hew thinks that Phanerozoic climate is primarily governed by cosmic rays. I am not a climatologist, so I can’t really get into the nitty-gritty details. Additionally, it was a fairly qualitative presentation, so this reflection is similarly mushy. There was a fast one that he tried to pull, and there was an abundance of sketchy science that is worth repeating.
First of all, Professor Veizen did repeatedly state that he was not in favor of polluting, and that he thought it was a generally bad idea. But he also concluded that CO2 climate sensitivity was overestimated because it did not create a water vapor feedback. The trouble is, even if we accept his overall model, the application of it to anthropogenic climate change is not necessarily appropriate.
Professor Veizen’s thesis basically goes like this:
Cosmic rays encourage cloud formation, by increasing nucleation.
Cloud formation effects the hydrological cycle, by changing albedo and precipitation.
The hydrological cycle drives CO2 levels, by regulating CO2 sequestration by land plants.
So CO2, humidity, and albedo are all increasingly direct feedbacks from cosmic ray abundance.
Even if we accept this model for the Phanerozoic, it still doesn’t tell us anything about 21st century climate change, for the following reason:
It assumes that CO2 is a passive positive feedback- an amplifier of humidity and/or precipitation.
The problem is that we know the current CO2 increase is NOT due to a feedback; it is due to fossil fuel burning. Therefore, we are already operating outside of the Veizen model. Since his model assumes that CO2 reacts passively, this model cannot be used for predicting the results of a forced change on CO2 content, which is our current situation.
As for the presentation, the only really deceptive bit was when he said that the 1 W/m2 dry CO2 forcing was equivalent to the 1 W/m2 variation in solar irradiance. As realclimate people have occasionally pointed out, the CO2 acts on surface area, which for a sphere is 4 times the cross section (4pi*r2 vs pi*r2). So using W/m2 on effects that operate over different numbers of square meters is dishonest. Interestingly, none of the 60 or so professional geoscientists in the audience called him on this.
The main criticism of the talk from our paleoclimate people was that he showed a lot of correlations of normalized, detrended data where his residual wiggles were way smaller than the magnitude of the detrending. For example, he showed a 1 per mil d18O curve without mentioning that the detrending removed 10 per mil of change.
My main problem was the lack of quantification of processes. For example, he was very coy about how much longer (or shorter) it took a cloud to nucleate in a high radiation vs. low radiation environment. Is it seconds, or hours, or years?
Furthermore, he did not discuss the magnitudes of changes that he claimed to observe on different timescales. For example, his observations on the 100 Ma timescale had 10 degree changes, while his observations on the century and decadal timescale were a degree or less. He correlated these with cosmic ray wiggles, but the magnitude of those cosmic wiggles on the various timescales was not linked to the magnitude of the climactic changes.
Finally, he failed to address any of the non-CO2 alternative hypotheses generally invoked to explain long term changes in paleoclimate. In order to get a new theory accepted, it is generally necessary to find a flaw in the current state of knowledge. He never mentioned the effect of traditional long timescale climate drivers like tectonics.
It was an interesting theory, and one worth trying to quantify, but running to the New York Times might have been a little bit premature.
First of all, Professor Veizen did repeatedly state that he was not in favor of polluting, and that he thought it was a generally bad idea. But he also concluded that CO2 climate sensitivity was overestimated because it did not create a water vapor feedback. The trouble is, even if we accept his overall model, the application of it to anthropogenic climate change is not necessarily appropriate.
Professor Veizen’s thesis basically goes like this:
Cosmic rays encourage cloud formation, by increasing nucleation.
Cloud formation effects the hydrological cycle, by changing albedo and precipitation.
The hydrological cycle drives CO2 levels, by regulating CO2 sequestration by land plants.
So CO2, humidity, and albedo are all increasingly direct feedbacks from cosmic ray abundance.
Even if we accept this model for the Phanerozoic, it still doesn’t tell us anything about 21st century climate change, for the following reason:
It assumes that CO2 is a passive positive feedback- an amplifier of humidity and/or precipitation.
The problem is that we know the current CO2 increase is NOT due to a feedback; it is due to fossil fuel burning. Therefore, we are already operating outside of the Veizen model. Since his model assumes that CO2 reacts passively, this model cannot be used for predicting the results of a forced change on CO2 content, which is our current situation.
As for the presentation, the only really deceptive bit was when he said that the 1 W/m2 dry CO2 forcing was equivalent to the 1 W/m2 variation in solar irradiance. As realclimate people have occasionally pointed out, the CO2 acts on surface area, which for a sphere is 4 times the cross section (4pi*r2 vs pi*r2). So using W/m2 on effects that operate over different numbers of square meters is dishonest. Interestingly, none of the 60 or so professional geoscientists in the audience called him on this.
The main criticism of the talk from our paleoclimate people was that he showed a lot of correlations of normalized, detrended data where his residual wiggles were way smaller than the magnitude of the detrending. For example, he showed a 1 per mil d18O curve without mentioning that the detrending removed 10 per mil of change.
My main problem was the lack of quantification of processes. For example, he was very coy about how much longer (or shorter) it took a cloud to nucleate in a high radiation vs. low radiation environment. Is it seconds, or hours, or years?
Furthermore, he did not discuss the magnitudes of changes that he claimed to observe on different timescales. For example, his observations on the 100 Ma timescale had 10 degree changes, while his observations on the century and decadal timescale were a degree or less. He correlated these with cosmic ray wiggles, but the magnitude of those cosmic wiggles on the various timescales was not linked to the magnitude of the climactic changes.
Finally, he failed to address any of the non-CO2 alternative hypotheses generally invoked to explain long term changes in paleoclimate. In order to get a new theory accepted, it is generally necessary to find a flaw in the current state of knowledge. He never mentioned the effect of traditional long timescale climate drivers like tectonics.
It was an interesting theory, and one worth trying to quantify, but running to the New York Times might have been a little bit premature.
Monday, November 06, 2006
Survivor: Laboratory
A while ago, a bunch of science promoters were suggesting that perhaps Hollywood should fund an LA Lab style TV drama, where hot, fit, lab scientists went out and solved the mysteries of the universe in ways that were way more exciting, dramatic, and tangible than real science is. The idea was to make science as misrepresented as law or medicine, when it came to television/ real world dissonance.
I think these suggestions are too high-brow. I think instead, they should redefine the common denominator with Survivor: Laboratory. Instead of an exotic jungle with silly adventure camp stunts thrown in, this would be rows of benches, test tubes, and occasionally combustible chemicals. Each team who first successfully synthesizes the correct compound gets immunity. Every show, the most triple thumbed, block-headed, OSHA hazard would get voted off. The protocol is repeated until there is only one survivor, who wins a 4 year scholarship to whatever school they can get into.
Like the current survivor, the show would be dominated not by the chemical/mechanical theory, but by the scheming, double crossing, and sabotaging that generates the most human interest. In this way, it would be very similar to actual professional scientific research. In fact, there could even be an episode where the teams have to crawl through a lab window and an obstacle course of dangerous glassware to steal Rosalind Franklin’s X-ray diffraction patterns.
Such a TV show could be relatively cheap to produce, entertaining, educational, and almost impossible to market. But those constraints didn’t stop Firefly or Operatunity, did they?
I think these suggestions are too high-brow. I think instead, they should redefine the common denominator with Survivor: Laboratory. Instead of an exotic jungle with silly adventure camp stunts thrown in, this would be rows of benches, test tubes, and occasionally combustible chemicals. Each team who first successfully synthesizes the correct compound gets immunity. Every show, the most triple thumbed, block-headed, OSHA hazard would get voted off. The protocol is repeated until there is only one survivor, who wins a 4 year scholarship to whatever school they can get into.
Like the current survivor, the show would be dominated not by the chemical/mechanical theory, but by the scheming, double crossing, and sabotaging that generates the most human interest. In this way, it would be very similar to actual professional scientific research. In fact, there could even be an episode where the teams have to crawl through a lab window and an obstacle course of dangerous glassware to steal Rosalind Franklin’s X-ray diffraction patterns.
Such a TV show could be relatively cheap to produce, entertaining, educational, and almost impossible to market. But those constraints didn’t stop Firefly or Operatunity, did they?
Friday, November 03, 2006
Vegemites are terrorists
According to various News Limited papers and websites, US Customs officials have banned the importation of vegemite from Australia to the USA. Evidently the reason given is that vegemite contains folate, which under US regulations can only be added to bread or cereal. Adding it to a spread used on bread is evidently a problem. If true (and with News Limited, you never know), then this has grave consequences for the US-Australia relationship.
First of all, why folate? The most well-known property of this substance is that it prevents neural tube defects in developing embryos. Are birth defects now a constitutional right? Wouldn’t a ban on harmful substances, like saturated fat or cane sugar, be more sensible? Or would those infringe on the right to be a fat slob?
Secondly, what about the free trade agreement? At no point during the FTA negotiations last year did anyone mention using FDA technicalities to block the exchange of culturally significant foodstuffs. This looks like protectionism, pure and simple.
Finally, imagine the consequences of the potential trade war that could erupt as a result. Australia could ban peanut butter, on the grounds that it is a potential allergen. It could forbid the import of Dr. Pepper, because that shit is nasty. And if the Australians wanted a truly disproportionate response, they could outlaw the baking of apple pie, as it has been correlated with dangerous levels of mindless militaristic jingoism*.
And that, my friends, would be a tragedy.
* I don’t know what foodstuffs promote thoughtful pacific jingoism, but anyone with a lead is welcome to comment.
First of all, why folate? The most well-known property of this substance is that it prevents neural tube defects in developing embryos. Are birth defects now a constitutional right? Wouldn’t a ban on harmful substances, like saturated fat or cane sugar, be more sensible? Or would those infringe on the right to be a fat slob?
Secondly, what about the free trade agreement? At no point during the FTA negotiations last year did anyone mention using FDA technicalities to block the exchange of culturally significant foodstuffs. This looks like protectionism, pure and simple.
Finally, imagine the consequences of the potential trade war that could erupt as a result. Australia could ban peanut butter, on the grounds that it is a potential allergen. It could forbid the import of Dr. Pepper, because that shit is nasty. And if the Australians wanted a truly disproportionate response, they could outlaw the baking of apple pie, as it has been correlated with dangerous levels of mindless militaristic jingoism*.
And that, my friends, would be a tragedy.
* I don’t know what foodstuffs promote thoughtful pacific jingoism, but anyone with a lead is welcome to comment.
Wednesday, November 01, 2006
Tuesday, October 31, 2006
A slippery situation
I realize that Canberra can be an intimidating city for people who grew up in non-synthetic communities. Despite the size, it is a quiet place. Down town is often dead quiet shortly after dark. The dispersed design of the city can discourage socializing, especially during the cold months of winter. And many people keep to themselves, their families, or their close networks of friends. However, none of this is an excuse for antisocial behavior.
Will the person who nicked the jars of Vaseline from the polishing lab please knock it off? I too have been single in Canberra, so I appreciate your situation. But trying to prepare an epoxy mount only to spend the afternoon searching the lab and heading down to storage is a pain in the butt. Used for its proper laboratory purpose, each one of those jars should last about 4 years. So you’ve swiped over a decade’s worth this month.
No, I’m not asking for you to return these things. Feel free to celebrate with your ill-gotten gain. But please don’t pilfer any more.
Will the person who nicked the jars of Vaseline from the polishing lab please knock it off? I too have been single in Canberra, so I appreciate your situation. But trying to prepare an epoxy mount only to spend the afternoon searching the lab and heading down to storage is a pain in the butt. Used for its proper laboratory purpose, each one of those jars should last about 4 years. So you’ve swiped over a decade’s worth this month.
No, I’m not asking for you to return these things. Feel free to celebrate with your ill-gotten gain. But please don’t pilfer any more.
Saturday, October 28, 2006
Is this a challenge I see before me?
I opened my door today to find a gleaming metal hand lying in the middle of the doormat. At first I thought it must have been severed from the arm of a robot. I don’t know many robots, but I suppose that this particular one could have been a light-fingered android from an automatonic society that practices Sharia law. Why it had been caught and punished on my front door was beyond me, but you can never be too sure these days.
So I picked up the hand, to get a closer view. Were the fingers electronic or hydraulic? Was it controlled by fiber-optic or wires? A brief inspection showed that it was in fact a shell. It was hollow on the inside, like a mitten or a glove. This was not a robotic hand at all. It was actually a glove made of steel. A gauntlet. And I had picked it up.
The trouble with picking up a gauntlet, even accidentally, is that once you have one in your hands, you can’t really put it back down again. It just isn’t done. So I had no choice but to determine what the challenge associated with the gauntlet might be. And the challenge is this.
Now, your ordinary man on the street might rightly think that writing a novel in 30 days is a crazy idea. But for me it is more than that. You see, I’m dyslexic. And not just a little bit dyslexic, either. I am absolutely completely fucking hopeless dyslexic. When driving, I don’t allow people to give me right or left directions, because it creates a traffic hazard the 50% of the time I turn the wrong way. It took me four goes and five years to pass my Australian car driving test as a result (the motorcycle test, which has no verbal instructions, was a cakewalk, even in a 4 degree mid-winter downpour).
Needless to say, writing my PhD thesis was a bit traumatic. It was worse than getting knocked off my motorcycle by a speeding Subaru. It was worse than getting interrogated on the Guyana/Brazil border by Polícia Federal who thought I was smuggling drugs (silly cops- geologists smuggle diamonds and gold, not drugs). But I’m getting a little bit tired of being afraid of words.
The best way to conquer a fear is to confront it. That’s one of the reasons I started this blog. It made me try to write complete paragraphs that were not spastic incoherencies. But I think I’ve gotten to the point where dipping a toe in the lake three times a week isn’t doing much for me anymore. I need to sink or swim.
If you’re scared of heights, jump out of an airplane without a parachute. If you’re scared of water, paddle a kayak over Niagra falls. If you’re scared of complete sentences, write a novel. In thirty days. If anyone wants to scrape me off the rocks at the end of the month, there’s a spatula in the kitchen.
So I picked up the hand, to get a closer view. Were the fingers electronic or hydraulic? Was it controlled by fiber-optic or wires? A brief inspection showed that it was in fact a shell. It was hollow on the inside, like a mitten or a glove. This was not a robotic hand at all. It was actually a glove made of steel. A gauntlet. And I had picked it up.
The trouble with picking up a gauntlet, even accidentally, is that once you have one in your hands, you can’t really put it back down again. It just isn’t done. So I had no choice but to determine what the challenge associated with the gauntlet might be. And the challenge is this.
Now, your ordinary man on the street might rightly think that writing a novel in 30 days is a crazy idea. But for me it is more than that. You see, I’m dyslexic. And not just a little bit dyslexic, either. I am absolutely completely fucking hopeless dyslexic. When driving, I don’t allow people to give me right or left directions, because it creates a traffic hazard the 50% of the time I turn the wrong way. It took me four goes and five years to pass my Australian car driving test as a result (the motorcycle test, which has no verbal instructions, was a cakewalk, even in a 4 degree mid-winter downpour).
Needless to say, writing my PhD thesis was a bit traumatic. It was worse than getting knocked off my motorcycle by a speeding Subaru. It was worse than getting interrogated on the Guyana/Brazil border by Polícia Federal who thought I was smuggling drugs (silly cops- geologists smuggle diamonds and gold, not drugs). But I’m getting a little bit tired of being afraid of words.
The best way to conquer a fear is to confront it. That’s one of the reasons I started this blog. It made me try to write complete paragraphs that were not spastic incoherencies. But I think I’ve gotten to the point where dipping a toe in the lake three times a week isn’t doing much for me anymore. I need to sink or swim.
If you’re scared of heights, jump out of an airplane without a parachute. If you’re scared of water, paddle a kayak over Niagra falls. If you’re scared of complete sentences, write a novel. In thirty days. If anyone wants to scrape me off the rocks at the end of the month, there’s a spatula in the kitchen.
Thursday, October 26, 2006
Tactile computing
Life in the lab is just humming along this month. I think I’ve finally licked the alkali problem, but we won’t get to test until sometime next month. Ultra low-level alkali measurements just don’t interest that many people.
I’ve also been spending time running SHRIMP I, the original Sensitive, High-Resolution Ion Micro Probe. SI is a grand old machine, built before the era of modern computing. It is manually controlled. Instead of clicking on a flat screen, it uses dials and wheels and knobs for everything. Opening the sample lock requires spinning a huge valve crank with both hands, reminiscent of diving in a U-boat, or operating a 19th century steam engine.
One of the problems with the computer age is that computer interfaces are so anti-tactile. You just click stuff on screens. There is no texture, no clutching or smashing or tasting, like in the rest of geology. Visualization only goes so far in a science where grasping concepts and feeling out hypotheses is so important.
Of course, it would be unblog-like for me to simply complain about something in this medium without attempting a solution. So I will try to interface my computer with my rock hammer. A tap seems to have no effect, but if I take a hefty swing, then-
I’ve also been spending time running SHRIMP I, the original Sensitive, High-Resolution Ion Micro Probe. SI is a grand old machine, built before the era of modern computing. It is manually controlled. Instead of clicking on a flat screen, it uses dials and wheels and knobs for everything. Opening the sample lock requires spinning a huge valve crank with both hands, reminiscent of diving in a U-boat, or operating a 19th century steam engine.
One of the problems with the computer age is that computer interfaces are so anti-tactile. You just click stuff on screens. There is no texture, no clutching or smashing or tasting, like in the rest of geology. Visualization only goes so far in a science where grasping concepts and feeling out hypotheses is so important.
Of course, it would be unblog-like for me to simply complain about something in this medium without attempting a solution. So I will try to interface my computer with my rock hammer. A tap seems to have no effect, but if I take a hefty swing, then-
Tuesday, October 24, 2006
Election time 2 (Senate)
Incumbent: Robert Menendez (D)
Challenger: Thomas Kean Jr. (R)
Third Party candidates: the usual mix.
The incumbent was appointed to the senate seat vacated by billionaire John Corzine. Corzine left the Senate to become Governor, replacing democrat Richard Codey, who assumed the position after Governor Jim McGreevey resigned following a corruption scandal (giving his lover a government position despite not having the requisite qualifications). But the democrats’ predilection for avoiding elections whenever possible is not on the ballot this year; senator Menendez is.
And the senator has an appalling record on tax, free trade, and civil liberties. A typical partisan democrat, he seems to favor governmental intervention almost everywhere. And while he supports party-line democratic freedoms like abortion and gay rights, his record on universal freedoms, as opposed to interest group freedoms, is lacking. When push came to shove on votes that pitched basic democratic principles against populism, he caved, supporting the Military commission vote and the flag desecration amendment.
His opponent is the State Senator Thomas Kean Jr. I generally don’t like dynastic politicians (his Dad was the legendary NJ Governor and 9-11 commission chairman), but in his limited time in the state legislature, Kean Jr. has shown an ability to protect the environment, avoid partisanship, and fight corruption. I reckon he deserves a chance in the big leagues.
For New Jersey’s Senate race, I support Thomas Kean Jr. (R)
Challenger: Thomas Kean Jr. (R)
Third Party candidates: the usual mix.
The incumbent was appointed to the senate seat vacated by billionaire John Corzine. Corzine left the Senate to become Governor, replacing democrat Richard Codey, who assumed the position after Governor Jim McGreevey resigned following a corruption scandal (giving his lover a government position despite not having the requisite qualifications). But the democrats’ predilection for avoiding elections whenever possible is not on the ballot this year; senator Menendez is.
And the senator has an appalling record on tax, free trade, and civil liberties. A typical partisan democrat, he seems to favor governmental intervention almost everywhere. And while he supports party-line democratic freedoms like abortion and gay rights, his record on universal freedoms, as opposed to interest group freedoms, is lacking. When push came to shove on votes that pitched basic democratic principles against populism, he caved, supporting the Military commission vote and the flag desecration amendment.
His opponent is the State Senator Thomas Kean Jr. I generally don’t like dynastic politicians (his Dad was the legendary NJ Governor and 9-11 commission chairman), but in his limited time in the state legislature, Kean Jr. has shown an ability to protect the environment, avoid partisanship, and fight corruption. I reckon he deserves a chance in the big leagues.
For New Jersey’s Senate race, I support Thomas Kean Jr. (R)
Election time (House of reps)
As an absentee voter, I will be voting this week. So here are my thoughts. But first, a caveat:
A while ago, the Bad Astronomer got criticized for posting some political thoughts on his blog. I have two words for such critics:
Bite me.
Participatory democracy (and really, is there any other kind?) is dependent on citizens communicating their thoughts in the leadup to an election. It is true that I am not a paid political pundit. But I am a voter, and thus it is my responsibility to look at the candidates, consider their strengths and weaknesses, and choose between them. If you have a problem with this, move to China. If you agree with the principle, but you think my choices are daft, feel free to comment. ‘tis the season.
House of Representatives (NJ district 12)
Incumbent: Rush Holt (D)
Challenger: Joe Sinagra (R)
Third party candidates: none
The incumbent has sounded increasingly like a Democratic party hack as his time in Washington has increased. Still, before entering politics, he was a nuclear physicist. While I disagree with his occasional forays into identity politics and partisan sniping, I think that having a congressman who actually understands how nuclear weapons are built is an obvious asset to the nation these days.
The challenger is an Air Force veteran and small business owner. While there is nothing immediately discouraging about him, he doesn’t seem to grasp the subtlety of complex issues, and his anti-immigrant stance appears to border on the extreme.
For New Jersey’s 12th district, I support Rush Holt (D).
(Senate tomorrow)
A while ago, the Bad Astronomer got criticized for posting some political thoughts on his blog. I have two words for such critics:
Bite me.
Participatory democracy (and really, is there any other kind?) is dependent on citizens communicating their thoughts in the leadup to an election. It is true that I am not a paid political pundit. But I am a voter, and thus it is my responsibility to look at the candidates, consider their strengths and weaknesses, and choose between them. If you have a problem with this, move to China. If you agree with the principle, but you think my choices are daft, feel free to comment. ‘tis the season.
House of Representatives (NJ district 12)
Incumbent: Rush Holt (D)
Challenger: Joe Sinagra (R)
Third party candidates: none
The incumbent has sounded increasingly like a Democratic party hack as his time in Washington has increased. Still, before entering politics, he was a nuclear physicist. While I disagree with his occasional forays into identity politics and partisan sniping, I think that having a congressman who actually understands how nuclear weapons are built is an obvious asset to the nation these days.
The challenger is an Air Force veteran and small business owner. While there is nothing immediately discouraging about him, he doesn’t seem to grasp the subtlety of complex issues, and his anti-immigrant stance appears to border on the extreme.
For New Jersey’s 12th district, I support Rush Holt (D).
(Senate tomorrow)
Sunday, October 22, 2006
Hawaii earthquake
Western Geologist and Highly Allocthonous have already weighed in on this, but I figured I might want to float a highly speculative hypothesis. On Hawaii, most of the volcanism occurs on the southern half of the island, mainly from the Mona Loa and Kilauea volcanoes. Both of these volcanoes generally erupt from a series of NE/SW trending rifts- for the probable reason, see Western Geologists’ post on the regional stress field. If magma emplacement is causing substantial spreading in the southern part of the island, but not in the extinct north, then an accommodation transform fault may exist to allow that. Slip on such a fault would, as far as I can tell, be basically the right direction for the observed motion.

Hopefully the real seismologists will explain what actually happened at this week's GSA meeting. Is anyone going? Anyone? Bueller?

Hopefully the real seismologists will explain what actually happened at this week's GSA meeting. Is anyone going? Anyone? Bueller?
Wednesday, October 18, 2006
Name Game 3
I heard back from Thomson Scientific last week. The good news is that an actual human being got back to me. The problem is that he doesn’t seem to understand what the issue I complained about was. He writes:
“This is how the author names are currently appearing on Web of Science
and we processed them according to how the name would appear on the
source article. Let me know if I misunderstood.
Title: Zr budgets for metamorphic reactions, and the formation of zircon
from garnet breakdown
Author(s): Degeling H, Eggins S, Ellis DJ
Source: MINERALOGICAL MAGAZINE 65 (6): 749-758 DEC 2001
IDS Number: 501UV
Title: In situ U-Pb dating of zircon formed from retrograde garnet
breakdown during decompression in Rogaland, SW Norway
Author(s): Tomkins HS, Williams IS, Ellis DJ
Source: JOURNAL OF METAMORPHIC GEOLOGY 23 (4): 201-215 MAY 2005
IDS Number: 930BC”
I tried explaining again; we'll see if there is any reply.
previous posts on this issue:
Playing the Name Game
Google Scholar's reply
“This is how the author names are currently appearing on Web of Science
and we processed them according to how the name would appear on the
source article. Let me know if I misunderstood.
Title: Zr budgets for metamorphic reactions, and the formation of zircon
from garnet breakdown
Author(s): Degeling H, Eggins S, Ellis DJ
Source: MINERALOGICAL MAGAZINE 65 (6): 749-758 DEC 2001
IDS Number: 501UV
Title: In situ U-Pb dating of zircon formed from retrograde garnet
breakdown during decompression in Rogaland, SW Norway
Author(s): Tomkins HS, Williams IS, Ellis DJ
Source: JOURNAL OF METAMORPHIC GEOLOGY 23 (4): 201-215 MAY 2005
IDS Number: 930BC”
I tried explaining again; we'll see if there is any reply.
previous posts on this issue:
Playing the Name Game
Google Scholar's reply
Monday, October 16, 2006
Absentee ballots
Excuse the interruption, but have any of you other American expats out there had trouble or delays in getting abentee ballots? I usually get my in about 2 weeks, but it has been almost three so far. We have a nerw county commissioner, though, so maybe she just isn't up to speed yet.
I just wish that I didn't have to get up at 5:30 to call her office and inquire...
I just wish that I didn't have to get up at 5:30 to call her office and inquire...
Saturday, October 14, 2006
Supernovas, zinc mines, and North Korean nuclear blasts
At first glance, there is no obvious connection between zinc mining, neutron star formation, and nutty North Korean dictators. But a closer looks suggests that they may have something in common. And that something is the smallest object known to man.
The best way to determine whether or not the North Koreans actually detonated a nuclear weapon this week would be to detect a nuclear particle which is impossible to shield. One such particle is the neutrino. The problem with neutrinos that they are difficult to detect. But due to a stroke of luck, the Kamioka neutrino observatory, buried deep in an abandoned zinc mine in the Japanese Alps, just so happens to be located on the opposite side of the Sea of Japan as the North Korean test site.
If I was a qualitative blogger, I’d pat myself on the back for being so clever now, and go do something useful with my life. But since this is a science blog, I should at least try, in the roughest possible terms, to quantify this hypothesis. Is there any way to determine whether or not the Kamioka detector should be expected to pick up a nearby nuclear blast?
Addressing this problem could be tricky. On the one hand, I know jack-skittles about nuclear physics. On the other hand, I can look stuff up on the internet. So, taking the only two things I know about the Japanese neutrino detector, is it possible to estimate the sensitivity to a nuclear test.
First, the disclosure of those two things. I know that a recent paper used the neutrino detector to look at neutrinos produced by U and Th decay in the Earth’s interior. I also know that it detected the 1987 supernova in the Magellanic cloud.
Basically, this is a detection efficiency issue. If I can figure out the detection efficiency for a supernova, and then rescale that to the test, I can see how the number of expected neutrinos compares to the yield from the supernova.
The simplest test is this: to get a comparable number of counts, how much Pu do I need to fission?
Constant counts means that production/distance squared= constant for both the test and the supernova, assuming perfect transmission. If I can get the distances to both events, and the production for the supernova, then I should be able to calculate how much Pu I need to fission in North Korea.
So I need the following quantities
-distance from Kamioka to test site. (there’s a national geographic atlas on my shelf)
-distance from Kamioka to large Magellanic cloud (wikipedia)
-number of neutrinos produced in a supernova.
-number of neutrinos produced per unit mass of Pu fission.
I thought I would be able to simply look up the number of neutrinos in a supernova in wikipedia, but my searches of the obvious entries were fruitless. So if I want to know it, I will have to calculate it from first principles. Is that possible for an isotope chemist who knows squat about physics?
Well, wiki does tell me that the neutrinos are produced by the electron capture of protons in the Fe core of a giant star, as it transforms into a neutron star. How big a star? Rigel, in Orion, is given as an example of a blue supergiant. It is 20 solar masses. So if we simple assume that 20 solar masses of 56Fe transforms all of its protons into neutrons, the number of neutrinos produces is simply the starting number of protons. 20 solar masses of 56Fe worth of protons. A large, but tractable number.
The total number of nucleons in 20 solar masses is basically avagadro’s number times the mass in grams. In 56Fe, 46% of those are protons. So, using Wiki or google again, we have 6.0221415x1023 x 1.98x1030kg x 1000 g/kg x 20= ~2.4x1058 nucleons.
Holy leptons! I’ve never seen a number that big applied to physical science before. Astronomers are nuts. Of course, only 46% of them are protons- the rest are already neutrons, and thus don’t need to transform, but the total number of neutrinos emitted is still 1.1x1058.
Distance to supernova: 168,000 light years x 9.46x1012 km/lightyear = 1.59x1018 km. And I get to square it.
Distance to Kamioka from test site ~900 km.
Neutrinos generated at test site necessary to equal supernova flux in Kamioka: 7.68x1027
That’s big, but not outrageous. Avagadro’s number is working on our side now, so to get back to kilos (not grams) we can divide by 6.03x1026. In fact, we only need 5.8 kilos of nucleons to undergo beta decay in North Korea to produce an equivalent neutrino flux to the magellanic supernova. Depending on the bomb geometry, the total Pu needed for a bomb is in the 5-10 kg range.
So what fraction of Pu neutrons actually undergo beta decay? This is not a simple question to answer, but a rough estimate would suggest that it between 2 and 5%. For example, the net reaction 239Pu+n -> 90Zr + 147Sm + 3n requires eight beta decays. The neutrons will undergo beta decay in a vacuum, but in the real world, they are more likely to react with a nearby nucleus. The reaction product could either be stable or undergo further beta decay.
So in order to produce the neutrino flux equivalent to supernova 1987a, the North Koreans would have to fission between 110kg and 290 kg of Pu. This is about 2 orders of magnitude higher than what one would expect from a simple device, and 3 orders of magnitude larger than a dud.
Of course, the Kamiokande II detector used in 1987 has since been replaced by a bigger, better detector. And other factors, such as neutrino oscillation, neutrino energy, detection efficiencies between neutrinos and anti-neutrinos, and stuff I’ve never heard of have been ignored. But since the value is only a couple orders of magnitude short, it might be worth considering by someone less ignorant than I. So if I was a Japanese neutrino researcher, I’d lay down the line on the new nationalist PM. With a more sensitive detector, we might be able to call the North Koreans’ bluff.
Anyone with the tiniest smidgeon of knowledge in any of these subjects is welcome to tell me how wrong I am, and why.
The best way to determine whether or not the North Koreans actually detonated a nuclear weapon this week would be to detect a nuclear particle which is impossible to shield. One such particle is the neutrino. The problem with neutrinos that they are difficult to detect. But due to a stroke of luck, the Kamioka neutrino observatory, buried deep in an abandoned zinc mine in the Japanese Alps, just so happens to be located on the opposite side of the Sea of Japan as the North Korean test site.
If I was a qualitative blogger, I’d pat myself on the back for being so clever now, and go do something useful with my life. But since this is a science blog, I should at least try, in the roughest possible terms, to quantify this hypothesis. Is there any way to determine whether or not the Kamioka detector should be expected to pick up a nearby nuclear blast?
Addressing this problem could be tricky. On the one hand, I know jack-skittles about nuclear physics. On the other hand, I can look stuff up on the internet. So, taking the only two things I know about the Japanese neutrino detector, is it possible to estimate the sensitivity to a nuclear test.
First, the disclosure of those two things. I know that a recent paper used the neutrino detector to look at neutrinos produced by U and Th decay in the Earth’s interior. I also know that it detected the 1987 supernova in the Magellanic cloud.
Basically, this is a detection efficiency issue. If I can figure out the detection efficiency for a supernova, and then rescale that to the test, I can see how the number of expected neutrinos compares to the yield from the supernova.
The simplest test is this: to get a comparable number of counts, how much Pu do I need to fission?
Constant counts means that production/distance squared= constant for both the test and the supernova, assuming perfect transmission. If I can get the distances to both events, and the production for the supernova, then I should be able to calculate how much Pu I need to fission in North Korea.
So I need the following quantities
-distance from Kamioka to test site. (there’s a national geographic atlas on my shelf)
-distance from Kamioka to large Magellanic cloud (wikipedia)
-number of neutrinos produced in a supernova.
-number of neutrinos produced per unit mass of Pu fission.
I thought I would be able to simply look up the number of neutrinos in a supernova in wikipedia, but my searches of the obvious entries were fruitless. So if I want to know it, I will have to calculate it from first principles. Is that possible for an isotope chemist who knows squat about physics?
Well, wiki does tell me that the neutrinos are produced by the electron capture of protons in the Fe core of a giant star, as it transforms into a neutron star. How big a star? Rigel, in Orion, is given as an example of a blue supergiant. It is 20 solar masses. So if we simple assume that 20 solar masses of 56Fe transforms all of its protons into neutrons, the number of neutrinos produces is simply the starting number of protons. 20 solar masses of 56Fe worth of protons. A large, but tractable number.
The total number of nucleons in 20 solar masses is basically avagadro’s number times the mass in grams. In 56Fe, 46% of those are protons. So, using Wiki or google again, we have 6.0221415x1023 x 1.98x1030kg x 1000 g/kg x 20= ~2.4x1058 nucleons.
Holy leptons! I’ve never seen a number that big applied to physical science before. Astronomers are nuts. Of course, only 46% of them are protons- the rest are already neutrons, and thus don’t need to transform, but the total number of neutrinos emitted is still 1.1x1058.
Distance to supernova: 168,000 light years x 9.46x1012 km/lightyear = 1.59x1018 km. And I get to square it.
Distance to Kamioka from test site ~900 km.
Neutrinos generated at test site necessary to equal supernova flux in Kamioka: 7.68x1027
That’s big, but not outrageous. Avagadro’s number is working on our side now, so to get back to kilos (not grams) we can divide by 6.03x1026. In fact, we only need 5.8 kilos of nucleons to undergo beta decay in North Korea to produce an equivalent neutrino flux to the magellanic supernova. Depending on the bomb geometry, the total Pu needed for a bomb is in the 5-10 kg range.
So what fraction of Pu neutrons actually undergo beta decay? This is not a simple question to answer, but a rough estimate would suggest that it between 2 and 5%. For example, the net reaction 239Pu+n -> 90Zr + 147Sm + 3n requires eight beta decays. The neutrons will undergo beta decay in a vacuum, but in the real world, they are more likely to react with a nearby nucleus. The reaction product could either be stable or undergo further beta decay.
So in order to produce the neutrino flux equivalent to supernova 1987a, the North Koreans would have to fission between 110kg and 290 kg of Pu. This is about 2 orders of magnitude higher than what one would expect from a simple device, and 3 orders of magnitude larger than a dud.
Of course, the Kamiokande II detector used in 1987 has since been replaced by a bigger, better detector. And other factors, such as neutrino oscillation, neutrino energy, detection efficiencies between neutrinos and anti-neutrinos, and stuff I’ve never heard of have been ignored. But since the value is only a couple orders of magnitude short, it might be worth considering by someone less ignorant than I. So if I was a Japanese neutrino researcher, I’d lay down the line on the new nationalist PM. With a more sensitive detector, we might be able to call the North Koreans’ bluff.
Anyone with the tiniest smidgeon of knowledge in any of these subjects is welcome to tell me how wrong I am, and why.
Friday, October 13, 2006
North Korea seismology blog roundup
First of all, the blog round-up from around the geologic community:
Highly Allochthonous, Green Gabbro, and Western Geologist all have examples of seismographs and discussions of what they mean. Since geochemistry is about as far from seismology as one can get, I recommend reading them, not me.
Some arms control sites, such as this guy, have suggested that the test might be a dud or a fake, as so far no escaped radioactive material has been detected. Specifically, the issue of whether or not the test could have been faked tweaked my curiosity. Assuming a safe containment system, is there any way to tell whether a nuclear explosion actually occurred?
Obviously, the whole point of testing underground is to contain all the fission products and radiation generated by the blast. This generally works pretty well; the carbon 14 curve shows that after the cessation of atmospheric testing in the 60’s, the amount of anthropogenic 14C in the atmosphere began to decline sharply. So, if the alpha, beta, gamma, and neutrons from the test are all contained, is there anything else that might get out?
To be continued...
Highly Allochthonous, Green Gabbro, and Western Geologist all have examples of seismographs and discussions of what they mean. Since geochemistry is about as far from seismology as one can get, I recommend reading them, not me.
Some arms control sites, such as this guy, have suggested that the test might be a dud or a fake, as so far no escaped radioactive material has been detected. Specifically, the issue of whether or not the test could have been faked tweaked my curiosity. Assuming a safe containment system, is there any way to tell whether a nuclear explosion actually occurred?
Obviously, the whole point of testing underground is to contain all the fission products and radiation generated by the blast. This generally works pretty well; the carbon 14 curve shows that after the cessation of atmospheric testing in the 60’s, the amount of anthropogenic 14C in the atmosphere began to decline sharply. So, if the alpha, beta, gamma, and neutrons from the test are all contained, is there anything else that might get out?
To be continued...
Thursday, October 12, 2006
How to tell an earthquake from a nuclear explosion (2)
There were rumors and press releases vibrating through cyberspace yesterday, suggesting that another test occurred, that it might have been an earthquake, and that nobody had any idea what was going on.
Here is the report page for that event.
First, consider the location, shown on this map.
The event was East of Japan, and relatively distant from North Korea. In addition, the depth was approximately 30 km, or about 3 times deeper than the deepest man-made hole ever drilled.
To fully understand this event, however, we need to look at the historical context.
As is obvious from this figure, hundreds of similar quakes have occurred over the past 15 years. This earthquake is completely standard. The above link does show a great representation of the Japanese Benioff zone, however. As is shown by the color coding, the quakes in the downgoing slab increase in depth to the west. A rough schematic, using the same color coding as the USGS, is shown below.

Benioff zones are one of the classic indicators of plate tectonics, and thus are way more interesting than nuclear weapons.
Here is the report page for that event.
First, consider the location, shown on this map.
The event was East of Japan, and relatively distant from North Korea. In addition, the depth was approximately 30 km, or about 3 times deeper than the deepest man-made hole ever drilled.
To fully understand this event, however, we need to look at the historical context.
As is obvious from this figure, hundreds of similar quakes have occurred over the past 15 years. This earthquake is completely standard. The above link does show a great representation of the Japanese Benioff zone, however. As is shown by the color coding, the quakes in the downgoing slab increase in depth to the west. A rough schematic, using the same color coding as the USGS, is shown below.

Benioff zones are one of the classic indicators of plate tectonics, and thus are way more interesting than nuclear weapons.
Monday, October 09, 2006
Seismology of a nuclear test
The North Koreans claim to have tested a nuclear bomb at about 10:30 this morning (11:30am eastern Australia time). Can geology tell us anything about what actually happened? Let us see.
First stop, the comprehensive test ban treaty organization. In theory, they have a monitoring network designed to detect nuclear tests. Unfortunately, as a UN organization, their webpage is full of schemes and classifications and plans and protocols, and as of 6 this evening, there is no data, and no press announcement or other hint that anything happened.
The Geoscience Australia earthquake page is great for small quakes near Australia, but does not contain any information on any events today.
What about the evil, super-secret world-dominating Americans?
The USGS earthquake list shows a little orange square in NE north Korea. Clicking either it or the “M2.5 to 4+ earthquake list” gives the following quake listing:
Clicking the appropriate entry gives the summary page for that earthquake
So, what can we see? First, the depth of the earthquake is listed as 0 km. Most of the earthquakes in this region are very deep (see “historical seismicity” under the “maps” tag and look at the color scale). These deep quakes happen in the downgoing slab, as the old pacific plate subducts beneath Japan. Thus they are quite different than the zero depth M 4.2 event from this morning.
What else can we determine? In theory, an explosion should have an all-positive first motion tensor. Unfortunately, first motion tensors only seem to be calculated for quakes larger than M 5.0, so that test will need to wait until the North Koreans detonate a larger bomb. It might be possible to put a DIY tensor together by looking at the raw data, but I don’t know where that is archived.
Similarly, I seem to recall from my deep, dark past that explosions have a higher P/S wave ratio than normal quakes. But once again, this will require having the S wave data, and I don’t know where to find that, either. Hopefully some of the real seismologists who occasionally visit the lounge can give us some hints.
The only conclusion that I can draw is that there was a M4.2 event on land in North Korea with a zero depth, which was dissimilar to other seismicity in the area.
First stop, the comprehensive test ban treaty organization. In theory, they have a monitoring network designed to detect nuclear tests. Unfortunately, as a UN organization, their webpage is full of schemes and classifications and plans and protocols, and as of 6 this evening, there is no data, and no press announcement or other hint that anything happened.
The Geoscience Australia earthquake page is great for small quakes near Australia, but does not contain any information on any events today.
What about the evil, super-secret world-dominating Americans?
The USGS earthquake list shows a little orange square in NE north Korea. Clicking either it or the “M2.5 to 4+ earthquake list” gives the following quake listing:
| MAG | UTC TIME | LATdeg | LONdeg | DEPTHkm | Region |
|---|---|---|---|---|---|
| 4.2 | 2006/10/09 01:35:28 | 41.294 | 129.134 | 0.0 | NORTH KOREA |
Clicking the appropriate entry gives the summary page for that earthquake
So, what can we see? First, the depth of the earthquake is listed as 0 km. Most of the earthquakes in this region are very deep (see “historical seismicity” under the “maps” tag and look at the color scale). These deep quakes happen in the downgoing slab, as the old pacific plate subducts beneath Japan. Thus they are quite different than the zero depth M 4.2 event from this morning.
What else can we determine? In theory, an explosion should have an all-positive first motion tensor. Unfortunately, first motion tensors only seem to be calculated for quakes larger than M 5.0, so that test will need to wait until the North Koreans detonate a larger bomb. It might be possible to put a DIY tensor together by looking at the raw data, but I don’t know where that is archived.
Similarly, I seem to recall from my deep, dark past that explosions have a higher P/S wave ratio than normal quakes. But once again, this will require having the S wave data, and I don’t know where to find that, either. Hopefully some of the real seismologists who occasionally visit the lounge can give us some hints.
The only conclusion that I can draw is that there was a M4.2 event on land in North Korea with a zero depth, which was dissimilar to other seismicity in the area.
Sunday, October 08, 2006
The relativistic geography of Sydney
Mrs. Lemming and I were required to spend some time in Sydney last week. It was a bit of a culture shock. Here in Canberra, the loudest morning noises are the warbles of the magpies and the screeches of the cockatoos. Last Friday we were woken at 5 in the morning by the sound of the pre-rush trucking. But this interminable traffic is merely an expression of Sydney geography.
In order to fully explain the geography of Sydney, a few simple mathematical concepts must first be explained. Geophysicists like to break the Earth down into simple hypothetical shapes, which they then integrate back together to approximate reality. One of the simplest of these shapes is the infinite half-plane. The infinite half-plane is a plane with a single linear edge, which stretches out to infinity in all other directions.
In urban planning, there is a form of development that closely approximates the infinite half-plane. I first observed this flying into San Jose, CA one night. Looking out the window of the aircraft, I could see the blackness of the Coast Ranges cutting north towards San Fran. East of that, the lights of Silicon Valley stretched out forever in every direction. What I observed was an infinite half-suburb.
Of course, the geography of Sydney is more complicated than a simple infinite half-suburb. The only Australian city that fits that category is Perth. But Sydney can be adequately described as two infinite half-suburbs, back-to-back, with a scenic harbour in between.
The harbour is finite, memorable, and spectacular. But on either side, an unending continuum of suburban sprawl stretches to the outer limits of mathematical comprehension. It is an unending tangle of six lane roads, traffic jams, and noisy, crowded tract housing.
A classical observer or remote sensing specialist might hypothesize that two infinite quarter suburbs might more accurately describe the Sydney geography, due to the presence of the Pacific Ocean to the East. But to do so would be a mistake. To consider the Pacific Ocean as a finite, tractable boundary is to ignore a fundamental truth of Sydney geography and culture. The Pacific Ocean is an asymptotic limit, which compresses space and roads in the coastal zone to ensure that, although the ocean can always be approached, it will always lie just out of reach.
The ocean is to Sydney what the speed of light is to relativistic physics. In physics, space and time dilate as c is approached, so that the energy required for the final acceleration reaches infinity. In Sydney, the dilation occurs in space and money as the ocean is approached, so that a flat becomes infinitely small, and the cost becomes infinitely high at the water’s edge. This creates an idealized fantasy. The beachfront apartment, like faster-than-light travel, becomes an impossible dream which can be perceived by anyone, even though a mathematical proof demonstrates that it cannot possibly exist.
Thus, although Sydney appears to have an eastern edge from a satellite, from the perspective of a motorist in the 'burbs, this spatial compression insures that you can navigate one-way streets and roundabouts forever while traveling east, and never hope to reach the sea. From the driver’s point of view, the streets are infinite and unending in every direction.
In a way, the utter despair inflicted by this geography was a motivating factor. When we lived in Sydney, Mrs. Lemming and I were constantly dreaming up schemes to escape. While waiting for my visa to be approved, I even created a wormhole, allowing me to bypass the space/fund continuum and reach the waves of Manly Beach using only a bicycle and seven Xeroxed pages of street directories.
Now that we live in Canberra, we are comfortable enough to happily potter away at home for weeks at a time. So in that sense, it is no surprise that Sydney is the state’s economic powerhouse. The poor souls who live there have no choice but to toil continuously, just to avoid the complete despondency that their forlorn surroundings would inflict, if they were foolish enough to lift their heads from their cubicles, get in a car, and drive through the never-ending traffic.
In order to fully explain the geography of Sydney, a few simple mathematical concepts must first be explained. Geophysicists like to break the Earth down into simple hypothetical shapes, which they then integrate back together to approximate reality. One of the simplest of these shapes is the infinite half-plane. The infinite half-plane is a plane with a single linear edge, which stretches out to infinity in all other directions.
In urban planning, there is a form of development that closely approximates the infinite half-plane. I first observed this flying into San Jose, CA one night. Looking out the window of the aircraft, I could see the blackness of the Coast Ranges cutting north towards San Fran. East of that, the lights of Silicon Valley stretched out forever in every direction. What I observed was an infinite half-suburb.
Of course, the geography of Sydney is more complicated than a simple infinite half-suburb. The only Australian city that fits that category is Perth. But Sydney can be adequately described as two infinite half-suburbs, back-to-back, with a scenic harbour in between.
The harbour is finite, memorable, and spectacular. But on either side, an unending continuum of suburban sprawl stretches to the outer limits of mathematical comprehension. It is an unending tangle of six lane roads, traffic jams, and noisy, crowded tract housing.
A classical observer or remote sensing specialist might hypothesize that two infinite quarter suburbs might more accurately describe the Sydney geography, due to the presence of the Pacific Ocean to the East. But to do so would be a mistake. To consider the Pacific Ocean as a finite, tractable boundary is to ignore a fundamental truth of Sydney geography and culture. The Pacific Ocean is an asymptotic limit, which compresses space and roads in the coastal zone to ensure that, although the ocean can always be approached, it will always lie just out of reach.
The ocean is to Sydney what the speed of light is to relativistic physics. In physics, space and time dilate as c is approached, so that the energy required for the final acceleration reaches infinity. In Sydney, the dilation occurs in space and money as the ocean is approached, so that a flat becomes infinitely small, and the cost becomes infinitely high at the water’s edge. This creates an idealized fantasy. The beachfront apartment, like faster-than-light travel, becomes an impossible dream which can be perceived by anyone, even though a mathematical proof demonstrates that it cannot possibly exist.
Thus, although Sydney appears to have an eastern edge from a satellite, from the perspective of a motorist in the 'burbs, this spatial compression insures that you can navigate one-way streets and roundabouts forever while traveling east, and never hope to reach the sea. From the driver’s point of view, the streets are infinite and unending in every direction.
In a way, the utter despair inflicted by this geography was a motivating factor. When we lived in Sydney, Mrs. Lemming and I were constantly dreaming up schemes to escape. While waiting for my visa to be approved, I even created a wormhole, allowing me to bypass the space/fund continuum and reach the waves of Manly Beach using only a bicycle and seven Xeroxed pages of street directories.
Now that we live in Canberra, we are comfortable enough to happily potter away at home for weeks at a time. So in that sense, it is no surprise that Sydney is the state’s economic powerhouse. The poor souls who live there have no choice but to toil continuously, just to avoid the complete despondency that their forlorn surroundings would inflict, if they were foolish enough to lift their heads from their cubicles, get in a car, and drive through the never-ending traffic.
Thursday, October 05, 2006
A zircon that predates the universe 2
So, in the first episode, I decided to investigate the dread option two. It really doesn’t make sense. This is science, how could anything possibly go wrong? The whole point of SHRIMP is to make U/Pb geochronology simple enough for a trained monkey to perform. So what could the problem be?
Well, in theory, the zircon could contain common Pb, in addition to the radiogenic Pb that was measured. So, what happens to the results when I apply a common Pb correction? The date of 15.2 Ga drops to 15.1 Ga. And the error increases from 0.3 to 0.4. Next?
What else could happen? Well, the first rule of analysis is this: always make sure that the thing you’re measuring is actually what you think it is. For Th, there is no question. But what about Pb?
Pb is a trickier matter. Zirconium and Hafnium are so similar in chemistry that it took science 99 years to isolate Hf from Zr and identify it as a separate element. As a result, Zircon contains substantial Hf- generally 1-2%. Because SIMS creates numerous molecular species, it is worth investigating the possibility of a molecular interference, such as 174Hf16O2 on 206Pb, or 176Hf16O2 on 208Pb.
As it turns out, the whole reason for building SHRIMP back in the 70’s was to have an ion probe with enough mass resolution to separate these peaks. Hf and O both have higher binding energies, and therefore lower mass/amu, than does Pb. Specifically, 206Pb has a mass defect of -27mAMU, while 16O is -5.1 and 174Hf is -59.9. So the HfO2 peak should be 43.1 mAMU lighter. This is seen in figure 1, a mass scan taken on SHRIMP 1.

A few caveats about these figures. First of all, these are quick-and-dirty, low resolution mass scans, so SHRIMP-haters should not use these peak shapes as evidence that the machine is inherently imprecise. Secondly, these are all scans of the Temora standard, correctly centered on Pb, and not of the pre-universal grain. Thirdly, I know that the calibration of the X axis is off. Setting this is generally more trouble than it’s worth, as we measure peak positions relative to each other; the nominal “absolute” value is not all that important.
Anyway, as fig 1 shows, The HfO2 and Pb peaks are clearly resolvable. Furthermore, in this grain, the 206Pb peak is considerably larger than the HfO2 peak. This is not the case for the mass 208 peaks, as fig 2 shows.

There are two reasons for this. First, 176Hf is 33 times more abundant than 174Hf. Second, although the Th/U ratio of this zircon is greater than 0.3, Th has a much longer half-life, so relative to 206Pb, not much 208Pb has been produced in the 418 million year lifetime of this particular grain.
Thus, if the instrument was accidentally measuring HfO2 peaks instead of Pb peaks, one would expect to find very large Th ages, accompanied by modest 238U ages. That is exactly what was observed with the “pre-universal” grain. Unfortunately, the measured Th/HfO2 ratio is not very geologically useful.
As for how this happens, it is quite simple. The machine can be told to autocenter peaks, to allow it to correct for magnet instability. However, this centering assumes that the 206Pb peak will be larger than the HfO2 peak (such as is the case in fig1). If you have a young, or low U zircon, then the HfO2 peak may be larger. In such instances, the instrument will pick the Hf peak instead of the Pb peak, and the lab lemming who foolishly left centering on will get geologically meaningless results.
Anally observant readers will notice a discrepancy between my figures and my explanation. I won’t say what this problem is, but I will tell you this: The answer, in a single word, is:
YTTERBIUM!
Well, in theory, the zircon could contain common Pb, in addition to the radiogenic Pb that was measured. So, what happens to the results when I apply a common Pb correction? The date of 15.2 Ga drops to 15.1 Ga. And the error increases from 0.3 to 0.4. Next?
What else could happen? Well, the first rule of analysis is this: always make sure that the thing you’re measuring is actually what you think it is. For Th, there is no question. But what about Pb?
Pb is a trickier matter. Zirconium and Hafnium are so similar in chemistry that it took science 99 years to isolate Hf from Zr and identify it as a separate element. As a result, Zircon contains substantial Hf- generally 1-2%. Because SIMS creates numerous molecular species, it is worth investigating the possibility of a molecular interference, such as 174Hf16O2 on 206Pb, or 176Hf16O2 on 208Pb.
As it turns out, the whole reason for building SHRIMP back in the 70’s was to have an ion probe with enough mass resolution to separate these peaks. Hf and O both have higher binding energies, and therefore lower mass/amu, than does Pb. Specifically, 206Pb has a mass defect of -27mAMU, while 16O is -5.1 and 174Hf is -59.9. So the HfO2 peak should be 43.1 mAMU lighter. This is seen in figure 1, a mass scan taken on SHRIMP 1.

A few caveats about these figures. First of all, these are quick-and-dirty, low resolution mass scans, so SHRIMP-haters should not use these peak shapes as evidence that the machine is inherently imprecise. Secondly, these are all scans of the Temora standard, correctly centered on Pb, and not of the pre-universal grain. Thirdly, I know that the calibration of the X axis is off. Setting this is generally more trouble than it’s worth, as we measure peak positions relative to each other; the nominal “absolute” value is not all that important.
Anyway, as fig 1 shows, The HfO2 and Pb peaks are clearly resolvable. Furthermore, in this grain, the 206Pb peak is considerably larger than the HfO2 peak. This is not the case for the mass 208 peaks, as fig 2 shows.

There are two reasons for this. First, 176Hf is 33 times more abundant than 174Hf. Second, although the Th/U ratio of this zircon is greater than 0.3, Th has a much longer half-life, so relative to 206Pb, not much 208Pb has been produced in the 418 million year lifetime of this particular grain.
Thus, if the instrument was accidentally measuring HfO2 peaks instead of Pb peaks, one would expect to find very large Th ages, accompanied by modest 238U ages. That is exactly what was observed with the “pre-universal” grain. Unfortunately, the measured Th/HfO2 ratio is not very geologically useful.
As for how this happens, it is quite simple. The machine can be told to autocenter peaks, to allow it to correct for magnet instability. However, this centering assumes that the 206Pb peak will be larger than the HfO2 peak (such as is the case in fig1). If you have a young, or low U zircon, then the HfO2 peak may be larger. In such instances, the instrument will pick the Hf peak instead of the Pb peak, and the lab lemming who foolishly left centering on will get geologically meaningless results.
Anally observant readers will notice a discrepancy between my figures and my explanation. I won’t say what this problem is, but I will tell you this: The answer, in a single word, is:
YTTERBIUM!
Wednesday, October 04, 2006
Happy Birthday Mrs. Lemming
Today is Ann's Birthday, and she's been having a rough couple of weeks, so I'm sure she'd appreciate any wishes left well.
Tuesday, October 03, 2006
Name game update
Last month, I payed out a couple of scientific databases over their inability to deal with authors who change their names. After a reader suggested that I was unfairly picking on huge corporations by insulting them in a blog without giving right of reply, I emailed both Google scholar and Thompson scientific to see why their databases weren’t able to determine that an author who changes her surname is still the same person. I still haven’t heard anything from Thomson, but Google sent me this reply:
“Hello Chuck,
Thank you for your note. As you may have noticed, Google Scholar is in
beta, and we're currently working out some of the kinks. We appreciate
your bringing this to our attention, and we'll pass it on to our
engineering team. Thank you for your assistance in improving Google
Scholar.
Sincerely,
The Google Scholar Team”
“Hello Chuck,
Thank you for your note. As you may have noticed, Google Scholar is in
beta, and we're currently working out some of the kinks. We appreciate
your bringing this to our attention, and we'll pass it on to our
engineering team. Thank you for your assistance in improving Google
Scholar.
Sincerely,
The Google Scholar Team”
Thursday, September 28, 2006
Tsunami info
There was an earthquake-generated tsunami in between Samoa and Tonga on the Tonga trench this afternoon. No local damage has been reported to date, and the low amplitude (0.08m) should prevent any distal destruction.
Information on the earthquake can be found here:
http://earthquake.usgs.gov/eqcenter/eqinthenews/2006/usteaj/#summary
The tsunami bulletin can be found here:
http://www.prh.noaa.gov/ptwc/wmsg
Information on the earthquake can be found here:
http://earthquake.usgs.gov/eqcenter/eqinthenews/2006/usteaj/#summary
The tsunami bulletin can be found here:
http://www.prh.noaa.gov/ptwc/wmsg
Women in pseudoscience
I should add an explanatory note to the argument presented as “option 1” in “A zircon that predates the universe”. I mentioned that in order to be taken seriously by the scientific community, it is necessary to explain previously existing data, as well as one’s own.
The alternative, which I will designate 1a, is to ignore the judgment of the scientific community by joining the lunatic fringe.
The issues and challenges surrounding a career in quackology are no different than those of any other profession. I do not intend to discuss them all, so for this post I will focus on a relatively basic problem: gender representation.
Why are there so few women pseudoscientists?
There are numerous blogs devoted to the issues of women representation in mainstream science. Whether you are looking for a witty, reasoned, or angry approach, this topic is extensively discussed online. Not so with women in the fringe. Despite career prospects that in some cases may rival that of academic scientists, this remains a male dominated profession. So at the very least, a quick review of some possible explanations is in order.
1. Pipeline problem. This hypothesis suggests that there are few women shysters because they never take the basic high school training that is required. Women who take college preparatory courses and do independent research are in danger of missing the opportunities created by spending one’s afternoons selling used cars, real estate, and standards-approved laboratory equipment.
2. Hostile work environment. There is no doubt that extreme nut jobs can create a difficult work environment. The social acclimation of people who belive the face on Mars is watching them can be marginal, at best. The cultivation of cults, harems, cloned teenage heartthrobs, and blockheaded arguments can also dissuade women from a career in quackery.
3. Systemic bias. In my travels as a wild youth, I met several women who had a basic misunderstanding of the Earth sciences. However, they do not seem to have ever made the career jump from uninformed housewife to corporate shill. A widespread, subconscious reluctance to give female applicants the same benefit of the doubt (even where the doubt is a fiction designed to distract and deceive) as males could potentially be an explanation.
4. Biological differences. Some people, including Harvard administrators, may suggest that there is an innate biological difference that makes fakery easier for men than for women. The unspoken corollary to this law is that there is no point trying to recruit women into the pseudoscientific disciplines, because they have neither the inclination nor the ability to succeed there. I suspect that the proponents of this particular hypothesis have all led sheltered, ivory tower lives that have never involved being wrapped around the finger of one of these professional manipulators. That, or they’re too up themselves to admit it.
Anyway, there are a few important take-home points here. First of all, this is a completely theoretical exercise. There is no possible relationship between the factors that govern bias in science and pseudoscience. The reason for this is obvious. Selection committees in the sciences are immune to charisma, charm, the hard sell, the flashy diversion, or any other pseudoscientific techniques. They only consider quantitative, verifiable factors when filling appointments. Always.
The alternative, which I will designate 1a, is to ignore the judgment of the scientific community by joining the lunatic fringe.
The issues and challenges surrounding a career in quackology are no different than those of any other profession. I do not intend to discuss them all, so for this post I will focus on a relatively basic problem: gender representation.
Why are there so few women pseudoscientists?
There are numerous blogs devoted to the issues of women representation in mainstream science. Whether you are looking for a witty, reasoned, or angry approach, this topic is extensively discussed online. Not so with women in the fringe. Despite career prospects that in some cases may rival that of academic scientists, this remains a male dominated profession. So at the very least, a quick review of some possible explanations is in order.
1. Pipeline problem. This hypothesis suggests that there are few women shysters because they never take the basic high school training that is required. Women who take college preparatory courses and do independent research are in danger of missing the opportunities created by spending one’s afternoons selling used cars, real estate, and standards-approved laboratory equipment.
2. Hostile work environment. There is no doubt that extreme nut jobs can create a difficult work environment. The social acclimation of people who belive the face on Mars is watching them can be marginal, at best. The cultivation of cults, harems, cloned teenage heartthrobs, and blockheaded arguments can also dissuade women from a career in quackery.
3. Systemic bias. In my travels as a wild youth, I met several women who had a basic misunderstanding of the Earth sciences. However, they do not seem to have ever made the career jump from uninformed housewife to corporate shill. A widespread, subconscious reluctance to give female applicants the same benefit of the doubt (even where the doubt is a fiction designed to distract and deceive) as males could potentially be an explanation.
4. Biological differences. Some people, including Harvard administrators, may suggest that there is an innate biological difference that makes fakery easier for men than for women. The unspoken corollary to this law is that there is no point trying to recruit women into the pseudoscientific disciplines, because they have neither the inclination nor the ability to succeed there. I suspect that the proponents of this particular hypothesis have all led sheltered, ivory tower lives that have never involved being wrapped around the finger of one of these professional manipulators. That, or they’re too up themselves to admit it.
Anyway, there are a few important take-home points here. First of all, this is a completely theoretical exercise. There is no possible relationship between the factors that govern bias in science and pseudoscience. The reason for this is obvious. Selection committees in the sciences are immune to charisma, charm, the hard sell, the flashy diversion, or any other pseudoscientific techniques. They only consider quantitative, verifiable factors when filling appointments. Always.
Tuesday, September 26, 2006
A zircon that predates the universe
I have on my desk a rather curious geochronology result. It is a SHRIMP analysis that I ran a month or two ago on a zircon- a very routine analysis. However, the result is somewhat surprising.
According to this summary sheet, the 206Pb/238U age of the zircon is 160 million years. Jurassic. No big deal.
The 207Pb/206Pb age, however, is a bit older- 3362 million years, or early Archean. That is a bit odd, but not completely implausible. A severe Pb-loss event caused by metamictization could, in theory, create such extreme discordance. The real problem is the thorium age.
The 208Pb/232Th age for this sample came out at 15,223 million years. 15.2 billion. That’s really old. It is more than three times the accepted age of the Earth and solar system (4.56 Ga), and considerably older than the best estimate that astronomers give us for the age of the universe: 13.7 Ga. Such an age is a bit counter-intuitive for a zircon from a Phanerozoic orogeny.
When trying to interpret such a result, the extreme value gives us only two possibilities:
1. The arcane disciplines of cosmochemistry, geochronology, isotopic analysis, and astronomy are all conspiratorial hoaxes perpetrated by godless, soul-destroying, ivory tower elitists, whose evil scheme is to avenge their social ostracism by polluting our precious bodily fluids.
2. I fucked up the analysis.
Obviously, choice one is more appealing to my sense of self-worth. It allows me to stroke my ego to sleep at night with the belief that I have single-handedly exposed the academic fraudsters. It fills me with the warm glow of knowing that humanity owes me for bringing justice, light, and honesty into the realm of physical science. It excites me that this selfless discovery will undoubtedly arouse the thousands of nubile young damsels who found zirconology fan clubs and fantasize about thorium decay.
Unfortunately, there is one small problem. There is a conceit among scientists- an unspoken rule of the laboratory code. It says that science should have predictive value. In order for my ground-breaking pre-universal zircon to overturn science, destroy the paradigm, and score me some hot chicks, I need to put it in a meaningful context. I can do this by either developing a new theorem, that will explain my data while reinterpreting the last 40 years of research in a new light, or I need to show that all the previous studies were flawed. Before I can do either, I need to understand the body of knowledge which I wish to overturn. This will require attending a library.
Thing is, I’m not real fond of libraries. The stacks smell musty, the babes give me dirty looks, and there is nothing to do other than sit and read. So instead of reviewing all the crusty old theories that my new data will disprove, I will procrastinate by testing hypothesis two, the outlandish possibility that the analysis is not completely perfect.
To be continued...
p.s. Note to the ANU students who read blogs instead of working on their mid-terms: Please don’t give the punch line away.
According to this summary sheet, the 206Pb/238U age of the zircon is 160 million years. Jurassic. No big deal.
The 207Pb/206Pb age, however, is a bit older- 3362 million years, or early Archean. That is a bit odd, but not completely implausible. A severe Pb-loss event caused by metamictization could, in theory, create such extreme discordance. The real problem is the thorium age.
The 208Pb/232Th age for this sample came out at 15,223 million years. 15.2 billion. That’s really old. It is more than three times the accepted age of the Earth and solar system (4.56 Ga), and considerably older than the best estimate that astronomers give us for the age of the universe: 13.7 Ga. Such an age is a bit counter-intuitive for a zircon from a Phanerozoic orogeny.
When trying to interpret such a result, the extreme value gives us only two possibilities:
1. The arcane disciplines of cosmochemistry, geochronology, isotopic analysis, and astronomy are all conspiratorial hoaxes perpetrated by godless, soul-destroying, ivory tower elitists, whose evil scheme is to avenge their social ostracism by polluting our precious bodily fluids.
2. I fucked up the analysis.
Obviously, choice one is more appealing to my sense of self-worth. It allows me to stroke my ego to sleep at night with the belief that I have single-handedly exposed the academic fraudsters. It fills me with the warm glow of knowing that humanity owes me for bringing justice, light, and honesty into the realm of physical science. It excites me that this selfless discovery will undoubtedly arouse the thousands of nubile young damsels who found zirconology fan clubs and fantasize about thorium decay.
Unfortunately, there is one small problem. There is a conceit among scientists- an unspoken rule of the laboratory code. It says that science should have predictive value. In order for my ground-breaking pre-universal zircon to overturn science, destroy the paradigm, and score me some hot chicks, I need to put it in a meaningful context. I can do this by either developing a new theorem, that will explain my data while reinterpreting the last 40 years of research in a new light, or I need to show that all the previous studies were flawed. Before I can do either, I need to understand the body of knowledge which I wish to overturn. This will require attending a library.
Thing is, I’m not real fond of libraries. The stacks smell musty, the babes give me dirty looks, and there is nothing to do other than sit and read. So instead of reviewing all the crusty old theories that my new data will disprove, I will procrastinate by testing hypothesis two, the outlandish possibility that the analysis is not completely perfect.
To be continued...
p.s. Note to the ANU students who read blogs instead of working on their mid-terms: Please don’t give the punch line away.
Sunday, September 24, 2006
A few quick notes:
On Friday, the last day of winter, we had a total fire ban. This does not bode well for summer.
I hope to write up a cool talk I went to see on the dating of Ethiopian hominid fossils, but this blog explains why they are interesting way more eloquently than anything I could write.
The Atlantic Hurricane season may be winding down, but you can still find updates here.
From the Bad Astronomer, a great update on Brown dwarfs, and a link to the homepage of Thierry Legault, who takes amazing astronomical pictures.
And finally, a search engine roundup:
Recently, I’ve been getting a statistically meaningful number (around 20-25%) of search requests for “Pluto conspiracy”.
Geologic mnemonics and various Bunsen burner queries are also represented more than once. As for the singletons, here are some highlights:
Donate a testicle to science
Australian nuclear weapons
Plausible deniability standard
Salt dough cockatoo
Duck egg cookies
Nitrogen trifecta system
what does it mean by when “only boys accepting feminism get kissed meaningfully”
steve irwin jfk diana
How intelligent are oysters?
steve irwin jfk diana
give me a catchy title for a science si unit lab
I hope to write up a cool talk I went to see on the dating of Ethiopian hominid fossils, but this blog explains why they are interesting way more eloquently than anything I could write.
The Atlantic Hurricane season may be winding down, but you can still find updates here.
From the Bad Astronomer, a great update on Brown dwarfs, and a link to the homepage of Thierry Legault, who takes amazing astronomical pictures.
And finally, a search engine roundup:
Recently, I’ve been getting a statistically meaningful number (around 20-25%) of search requests for “Pluto conspiracy”.
Geologic mnemonics and various Bunsen burner queries are also represented more than once. As for the singletons, here are some highlights:
Donate a testicle to science
Australian nuclear weapons
Plausible deniability standard
Salt dough cockatoo
Duck egg cookies
Nitrogen trifecta system
what does it mean by when “only boys accepting feminism get kissed meaningfully”
steve irwin jfk diana
How intelligent are oysters?
steve irwin jfk diana
give me a catchy title for a science si unit lab
Saturday, September 23, 2006
What is a Lab Lemming?
A Lab Lemming is a creature which mindlessly follows its protocols over the precipice of understanding, to drown in the frigid sea of uncertainty. Everyone who has ever fallen into research over their head, and gulped down the icy brine of confusion is welcome here. Grab a couch by the fire, dry off, warm up, and tell the tale of how your scientific hubris led you astray.
To proclaim a purpose or theme for The Lounge would be to deny its empirical nature. This blog is basically a collection of thoughts about working in a scientific lab, with a focus on the nitty gritty details of collecting data that is scientifically useful.
In my opinion, mainstream scientific education and commentary often fall into a number of pitfalls that reduce their effectiveness. These include, but are not limited to: solemnity, condescension, formalism, frigidity, politeness, hero-worship, and generalization. Thus, I will try to make this blog as silly, challenging, relaxed, visceral, rude, irreverent, and specific as possible.
It may be that my jokes are so esoteric that only ten people on the planet understand them (and only two find them amusing). It may be that semi-erotic descriptions of the large ion lithophiles are not the most appropriate way to teach analytical techniques. But the heart of the scientific endeavour is experimentation. And if I don’t worship oysters and Bunsen burners, ponder mutant rats, defend Pluto, or collect rude mnemonics, who will?
To proclaim a purpose or theme for The Lounge would be to deny its empirical nature. This blog is basically a collection of thoughts about working in a scientific lab, with a focus on the nitty gritty details of collecting data that is scientifically useful.
In my opinion, mainstream scientific education and commentary often fall into a number of pitfalls that reduce their effectiveness. These include, but are not limited to: solemnity, condescension, formalism, frigidity, politeness, hero-worship, and generalization. Thus, I will try to make this blog as silly, challenging, relaxed, visceral, rude, irreverent, and specific as possible.
It may be that my jokes are so esoteric that only ten people on the planet understand them (and only two find them amusing). It may be that semi-erotic descriptions of the large ion lithophiles are not the most appropriate way to teach analytical techniques. But the heart of the scientific endeavour is experimentation. And if I don’t worship oysters and Bunsen burners, ponder mutant rats, defend Pluto, or collect rude mnemonics, who will?
Tuesday, September 19, 2006
Blogular hiatus
It has been a year since Mrs. Lemming and I bought our house. Our rate of renovating appears to resemble exponential decay, as the longer the work goes on, the slower the rate of progress. So I really need to put in some extra time to finish it off.
In addition, I recently had a look around the blogosphere, and found it fairly depressing. According to The Truth Laid Bear, most of the huge blog sites are partisan political propaganda. I kinda don’t really see the point of making esoteric jokes and explaining how stuff works to ten people a day, when a million times that number wolf down deliberate misinformation.
Finally, spring has sprung, and the number of pleasant outdoor hours has increased from three to 12.
So I’m taking a working holiday. I’m going to put my living room back together, paint it, and then put in a garden so that el Nino can desiccate it. This is not the end; I still have posts about paying for open access, zircons that predate the universe, and the terminology of lab culture in the works. I just don’t have the time or the interest in finishing them off right now.
Later, dudes.
In addition, I recently had a look around the blogosphere, and found it fairly depressing. According to The Truth Laid Bear, most of the huge blog sites are partisan political propaganda. I kinda don’t really see the point of making esoteric jokes and explaining how stuff works to ten people a day, when a million times that number wolf down deliberate misinformation.
Finally, spring has sprung, and the number of pleasant outdoor hours has increased from three to 12.
So I’m taking a working holiday. I’m going to put my living room back together, paint it, and then put in a garden so that el Nino can desiccate it. This is not the end; I still have posts about paying for open access, zircons that predate the universe, and the terminology of lab culture in the works. I just don’t have the time or the interest in finishing them off right now.
Later, dudes.
Saturday, September 16, 2006
Soft rock cat fight
According to RealClimate, The American Association of Petroleum Geologists and the American Quaternary Association have gotten their knickers in a twist about the recent decision by the AAPG to grant a journalism award to the novelist Michael Crichton.
Some random thoughts:
If writing novels about cloned dinosaurs and global warming hoaxery is an example of what the AAPG considers to be good science journalism, is it any surprise that oil costs $70 a barrel? How can anyone expect to keep exploration costs down when tarot cards and divining sticks cost so much these days?
By accusing the AAPG of self-interest, the AQA is totally and completely hypocritical. After all, the only possible reason to study the Quaternary is self-interest, since that is the period in which we happen to live.
So much love lost so quickly. It was only last century that Pleistocene paleontologists (and I don’t mean Neanderthals with doctorates in trilobitology; I mean mammoth-lovers) and petroleum geologists alike were swooning, hand-in-hand, at the richness of the California tar pits for both of their respective professions.
Given that this debate is between rival factions of soft rockers, I’m surprised that it has been so civilized. After all, they are actually using words. Correctly. Given the usual level of debate between environmental and ore geologists, I would have expected them to bare teeth, hurl feces, and howl at each other from opposing tree-tops.
Some random thoughts:
If writing novels about cloned dinosaurs and global warming hoaxery is an example of what the AAPG considers to be good science journalism, is it any surprise that oil costs $70 a barrel? How can anyone expect to keep exploration costs down when tarot cards and divining sticks cost so much these days?
By accusing the AAPG of self-interest, the AQA is totally and completely hypocritical. After all, the only possible reason to study the Quaternary is self-interest, since that is the period in which we happen to live.
So much love lost so quickly. It was only last century that Pleistocene paleontologists (and I don’t mean Neanderthals with doctorates in trilobitology; I mean mammoth-lovers) and petroleum geologists alike were swooning, hand-in-hand, at the richness of the California tar pits for both of their respective professions.
Given that this debate is between rival factions of soft rockers, I’m surprised that it has been so civilized. After all, they are actually using words. Correctly. Given the usual level of debate between environmental and ore geologists, I would have expected them to bare teeth, hurl feces, and howl at each other from opposing tree-tops.

