With the new northern school year underway, we can expect that the shorter days and colored leaved will bring with them a bevy of complaints by academic bloggers about the evils of Wikipedia and how venal students are for using it as a reference.
This does not strike me as constructive criticism. After all, Wikipedia is certainly no worse than any other encyclopedia, and it is an incredibly useful resource. So instead of whinging about it, I’m going to explain what I use it for, and when I don’t think it is much good. I challenge all the wikiwhinging professors out there to come clean and do the same.
I have two main uses for wikipedia: Looking up ‘common knowledge’, and giving myself a background on things I know nothing about.
An example of the first is the mass of Jupiter. This is a very well known constant- it is the main unit of mass for exoplanetary studies, for example. But I don’t know off the top of my head how many kg (or earth masses, which I do know) Jupiter is. Wikipedia is the easiest way to get this number, and the chances of it being wrong are quite small.
As far as the general knowledge about stuff I don’t understand, my approach is generally to read the article, then dig into the links at the end if I need to be sure of any particular facts, or need more depth. The last thing I looked up was the history of Guangzhou, and I didn’t follow it up because the interest was casual.
I would use Wikipedia for math and physics equations, except that I find it to be terribly obtuse and difficult to find simple equations or succinct descriptions.
I use the sites linked in the “useful links” part of the sidebar for technical information.
If I need to understand something in Wikipedia at a research level, I generally come up with some keywords based on the article and plug them into google scholar.
I find that Google scholar is better for keywords and titles, while georef is better for author or journal searches.
So, wikiwhingers, come clean. What do you use the masses encyclopedia for? (feel free to blog at length on your home site on this subject)
I'm a geochemist. My main interest is in-situ mass spectrometry, but I have a soft spot in my heart for thermodynamics, poetry, drillers, trees, bicycles, and cosmochemistry.
Wednesday, September 29, 2010
Monday, September 27, 2010
Monday, September 20, 2010
Why are minerals important?

The other day, someone (a non-geologist scientist) asked why minerals were important, and the earth can’t just be thought of as a homogenous lump of elements. Here in myopic subfield land, it sounds like a stupid question, but mineralogy and petrology do not exactly have the highest profile amount the general population. The man on the street thinks a petrologist is someone who works for Exxon. Word thinks the petrologist is a misspelling of pathologist. So I took a quick look at my old undergrad petrology textbook, and it doesn’t even consider the question. The introduction starts, “Igneous rocks are formed by the cooling and crystallization of magma, which is molten rock that”.
It then goes into definitions, and there is no attempt to even sell the field.
On a related note, when I left the ANU back in ’07, they were performing a curriculum review, and one of the ideas floated was to ditch mineralogy as a core required class. So even Earth Scientists (evidently ‘geologist’ is old fashioned, and creates insecurity among our colleagues who don’t actually know what a rock is) underappreciate the organization of atoms into ordered structures.
I got into petrology because I thought it was cool, and then strayed into geochem by inertia. But I’ve never really defended its importance before. So I’ll let all you lurkers speak up here. Consider this a hard rock version of the introduce yourself post. What is great about mineralogy and petrology, and how do you explain petrology in a sentence to someone who thinks you work for Exxon?
Friday, September 17, 2010
The lead isotope systematics of pregnancy and lactation
Determining calcium loss and lead exposure in pregnant women is not easy. Most of the bodies calcium is stored in bones, and because lead substitutes for calcium in most minerals (biogenic or otherwise), bone hydroxyapatite can also be a potential source for lead, if bone resorption occurs. But detecting this is not always easy.
Bone density measurements can be done by X-rays, but in pregnant women this risks giving the unborn child super powers. Bone biopsies are painful and intrusive. So isotope geochemistry is a much less harmful way of measuring whether or not bone resorption is occurring, and whether or not it is contributing lead to the bloodstream.
In the study reported in these papers, European immigrant women who became pregnant a few years after migrating to Australia were studied. This is important- soft tissues exchange lead rapidly with the environment, whole bone matter can take decade to equilibrate. Most environmental lead in Australia comes from the Proterozoic Mt. Isa and Broken Hill mines. These lie on (and help define) the earth isotopic evolution curve, so have a 208Pb/206Pb ratio of about 2.23. Most Eastern European women who grew up in the Eastern Bloc were primarily exposed to Paleozoic lead with an isotopic 208Pb/206Pb ratio of about 2.10. So their soft tissues and bones should have different isotopic signatures.
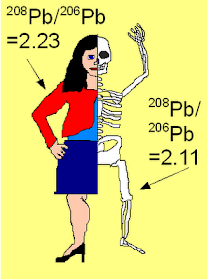
Figure 1. An Australian immigrant will have different Pb isotopic signatures in bones and soft tissues.
If such a person starts to resorb bone calcium, this should also liberate Pb substituting for calcium, and the blood Pb isotopic ratio should move towards the skeletal value. This is exactly what Gulson et al. saw during pregnancy and lactation in women who did not take calcium supplements.
Refs
B.L. Gulson, C.W. Jameson, K.R. Mahaffey, K.J. Mizon, M.J. Korsch, G. Vimpani; Pregnancy increases mobilization of lead from maternal skeleton; The Journal of Laboratory and Clinical Medicine, Volume 130, Issue 1, Pages 51-62 (July 1997)
GULSON B. L. ; MAHAFFEY K. R. ; JAMESON C. W. ; MIZON K. J. ; KORSCH M. J. ; CAMERON M. A. ; EISMAN J. A. ; Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy; The Journal of laboratory and clinical medicine 1998, vol. 131, no4, pp. 324-329
GULSON, B., MIZON, K., KORSCH, M., PALMER, J., & DONNELLY, J. (2003). Mobilization of lead from human bone tissue during pregnancy and lactation—a summary of long-term research The Science of The Total Environment, 303 (1-2), 79-104 DOI: 10.1016/S0048-9697(02)00355-8
* For what it's worth, every Russian bride I knew during the time period of this study had an advanced degree in physics, and was working in Australian academia or industry to win their family's bread.
Thursday, September 16, 2010
BHP calls for carbon tax
BHP, the largest mining company in Australia (and the world), is now calling for a carbon tax in Australia, according to various news sources. Like just about everyone else, I'm not really sure what to think of this, so read between the lines of the linked articles and see what insight might be gained. BHP is widely diversified, and has major holdings in export coal, export iron ore, oil & gas, uranium, aluminum, and various base and ferrous metals.
In the recent election, neither major party supported a price on carbon in the next three years, but the Greens forced the government to set up an investigative committee on the matter as a price of forming a governing coalition.
In the recent election, neither major party supported a price on carbon in the next three years, but the Greens forced the government to set up an investigative committee on the matter as a price of forming a governing coalition.
Saturday, September 11, 2010
Maybe Latin ain't such a bad idea
I hate Latin- it is one of the reasons I'm not a biologist. And I love the multicultural way in which geology incorporates technical terms from all around the world. Forgot your vocab? That's fine; the Norwegian sounding words are more likely to relate to ice than the Arabic ones. Still, there are a few terms that make me wonder. After the eruption earlier this year, everyone knows about Eyjafjallajökull. But at the opposite end of the time scale, Nuvvuagittuq ain't much easier. Of course, there are alternatives, as was demonstrated at post-conference beers:
Me:
How do you pronounce Noov- Nuff- Nuvatta- That really old metamorphic belt northeast of Hudson Bay?
Canadian professor:
It is pronounced "Porpoise Cove."
Me:
How do you pronounce Noov- Nuff- Nuvatta- That really old metamorphic belt northeast of Hudson Bay?
Canadian professor:
It is pronounced "Porpoise Cove."
Sunday, September 05, 2010
Chlorine á gogo
There is a Universe Today article on the lack of organics found by the Voyager missions, which suggests that the chlorinated organics originally thought to be cleaning products could have actually been reaction products from Martian organics and Martian perchlorates (perchlorates and organic molecules react vigorously when given the chance- see the Space Shuttle’s solid rocket boosters for an example). The paper is not yet available, and I’m not a big fan of the press-release-before-publication model that NASA seems to be getting fond of these days. So I’m gonna sandbag the study based on the interview.
They say,
So, measure the martian Cl isotopic ratio and see in the Viking measurement is terrestrial or martian.
This is not a well designed experiment. We know from Sharp et al. (2007) that Cl isotope ratios don’t vary much either on Earth or in chondrites. One permil is 1 tenth of a percent, so a 1 permil difference is a change in 35Cl/37Cl ratio from 3.125 to 3.128. It is unlikely that the Voyager measurements are anywhere close to precise enough to see this sort of variation.
Long et al. (1993) show that surface processes on Earth change the ratio by up to 4 permil. Nakamura et al. (2009) show a similar change in Cl metabolized by organochlorine-eating bacteria. The only place Cl isotopic variations exceed 1% (ten permil) is the moon (Sharp et al. 2010), and this is due to an extremely hydrogen-poor environment (insufficient H for chlorine to volatilize as HCl when outgassing from magma) that we know doesn’t exist on Mars, as it is covered in ice and hydrous minerals.
Luckily, we have Martian meteorites. They have lots of Cl in them (Bridges et al. 2001), so we should be able to get Cl isotopic ratios for phases present in those samples. I suspect that Sharp might be doing that as we speak. But there is no reason to expect that the values will be different enough to terrestrial that they can be used to test the provenance of the Viking Cl isotopic analyses.
They say,
“One reason the chlorinated organics found by Viking were interpreted as contaminants from Earth was that the ratio of two isotopes of chlorine in them matched the three-to-one ratio for those isotopes on Earth. The ratio for them on Mars has not been clearly determined yet. If it is found to be much different than Earth's, that would support the 1970s interpretation.”
So, measure the martian Cl isotopic ratio and see in the Viking measurement is terrestrial or martian.
This is not a well designed experiment. We know from Sharp et al. (2007) that Cl isotope ratios don’t vary much either on Earth or in chondrites. One permil is 1 tenth of a percent, so a 1 permil difference is a change in 35Cl/37Cl ratio from 3.125 to 3.128. It is unlikely that the Voyager measurements are anywhere close to precise enough to see this sort of variation.
Long et al. (1993) show that surface processes on Earth change the ratio by up to 4 permil. Nakamura et al. (2009) show a similar change in Cl metabolized by organochlorine-eating bacteria. The only place Cl isotopic variations exceed 1% (ten permil) is the moon (Sharp et al. 2010), and this is due to an extremely hydrogen-poor environment (insufficient H for chlorine to volatilize as HCl when outgassing from magma) that we know doesn’t exist on Mars, as it is covered in ice and hydrous minerals.
Luckily, we have Martian meteorites. They have lots of Cl in them (Bridges et al. 2001), so we should be able to get Cl isotopic ratios for phases present in those samples. I suspect that Sharp might be doing that as we speak. But there is no reason to expect that the values will be different enough to terrestrial that they can be used to test the provenance of the Viking Cl isotopic analyses.
Saturday, September 04, 2010
Choice magazine to consumers: don’t eat white dwarfs
There is a Choice Magazine report advocating for stoplight rating of foods, to help consumers eat more healthily. Their rating system uses units of 100g/mL (see figure 1, below). That is tenths of a kilogram per milliliter. I don’t know what they are eating, but the densest known substance at the surface of the Earth is osmium metal, with a density of about 22 g/mL, or 0.22 100g/mL.
Their “green light” value for sodium is 0.3 100g/mL, which is about 50% denser than anything on Earth. For comparison, a pure halite crystal 1 centimeter on a side (salt conveniently grows in cubes) will contain 2.16 g/cc x 0.39 g(Na)/g(total) = 0.85 g sodium. In units of 100g/ml, pure salt thus has a value of 0.0085 100g/mL.
Never-the-less, they rate almost every cereal as having an orange or red light rating.
There can be only one explanation. While osmium may be the densest material at surface pressures, at higher pressures many things can be more dense. As an example, consider a white dwarf star. A white dwarf is the burned out core of a star which has run out of hydrogen fuel and collapsed into a super dense state. Although calculating a diameter (and thus density) is not easy, they are generally thought to be about a million g/cc, or a ton/cc. On the Choice Magazine scale, that would weigh in at ten thousand 100g/cc.
Of course, white dwarfs are mostly carbon and oxygen, not sodium. But lets assume that they have a solar O/Na ratio. Using the Asplund et al. (2006) values, the solar O/Na ratio is about 300. But since white dwarves have carbon, silicon, etc. in them as well, we should really look at the ratio of everything except H and He to sodium. This is about 600 (in other words, carbon plus nitrogen plus all the heavier metals are about as abundant as oxygen).
So a white dwarf sodium content, using choice magazine units, is about 16.7 100g/mL.

That is more than ten times the 1.5 100g/mL “red light” value they suggest.
So eating degenerate matter from the cores of burned out stars is not recommended by Choice. It contains too much sodium, and might give you high blood pressure.
M. Asplund , N. Grevesse, A. J. Sauval; The solar chemical composition; Nuclear Physics A 777 1–4 (2006)
Their “green light” value for sodium is 0.3 100g/mL, which is about 50% denser than anything on Earth. For comparison, a pure halite crystal 1 centimeter on a side (salt conveniently grows in cubes) will contain 2.16 g/cc x 0.39 g(Na)/g(total) = 0.85 g sodium. In units of 100g/ml, pure salt thus has a value of 0.0085 100g/mL.
Never-the-less, they rate almost every cereal as having an orange or red light rating.
There can be only one explanation. While osmium may be the densest material at surface pressures, at higher pressures many things can be more dense. As an example, consider a white dwarf star. A white dwarf is the burned out core of a star which has run out of hydrogen fuel and collapsed into a super dense state. Although calculating a diameter (and thus density) is not easy, they are generally thought to be about a million g/cc, or a ton/cc. On the Choice Magazine scale, that would weigh in at ten thousand 100g/cc.
Of course, white dwarfs are mostly carbon and oxygen, not sodium. But lets assume that they have a solar O/Na ratio. Using the Asplund et al. (2006) values, the solar O/Na ratio is about 300. But since white dwarves have carbon, silicon, etc. in them as well, we should really look at the ratio of everything except H and He to sodium. This is about 600 (in other words, carbon plus nitrogen plus all the heavier metals are about as abundant as oxygen).
So a white dwarf sodium content, using choice magazine units, is about 16.7 100g/mL.

Figure 1. The traffic light rating table from the choice magazine report.
That is more than ten times the 1.5 100g/mL “red light” value they suggest.
So eating degenerate matter from the cores of burned out stars is not recommended by Choice. It contains too much sodium, and might give you high blood pressure.
M. Asplund , N. Grevesse, A. J. Sauval; The solar chemical composition; Nuclear Physics A 777 1–4 (2006)
Thursday, September 02, 2010
RIP Brian Welsh
Like many people who grew up in my hometown of West Windsor NJ, I was horrified that one of our West Windsor-Plainsboro High School teachers, Mr. Brian Welsh, committed suicide by train last weekend. Local newspaper reports can be found around the internet.
To say that Mr. Welsh was a great teacher is an understatement. He was a legend who made the Robin Williams character in “Dead Poets Society” look like a clock puncher.
When I was a student 20 years ago, Mr. Welsh taught a class on politics and law, called IPLE (Introduction to Political and Legal Experience). This is not a natural point of interest for teenage boys and girls. So the way in which Mr. Welsh sucked kids into his classes is something we could all learn from, even if few have the talent and passion to emulate the man.
The class was not required for anything, be it the district, the state, or any of the colleges to which students aspired. But it was a popular class, taken by everyone from the Ivy League aspirants to the kids on parole.
First, consider what he didn’t do. He didn’t sex his classes up. It was what it was, and there was no bait-and-switch or fancy dressing. He wasn’t a pushover; his classes appealed because they were involved, not breezy. He didn’t dumb it down. You had to work hard, write well, and get out and do all sorts of non-traditional activities. He didn’t hammer it in. There was no fear, or guilt, or condensation. The material was there to be learned by whoever was interested. Instead, Mr. Welsh motivated his students to learn.
I don’t know if I can really explain why he was such a great teacher. Certainly he knew his subject matter, and explained it passionately. His IPLE class requirements made it personal, though grading contracts, hands-on exercises and non-traditional requirements. I still remember a classmate of ours getting class credit for explaining his day in court where he beat a driving-with-suspended-license charge. Mr. Welsh used these features to connect what would normally be an austere, esoteric body of knowledge to our every day lives.
But most importantly, he gave the encouragement necessary to convince us kids that we could use this knowledge, and the applications of it, to make our way in the world. It isn’t that he showed us how to be lawyers or pundits; very few of us did (although those few have generally done quite well). The lesson was more fundamental than that. He inspired us how to combine our knowledge and our passions to set goals and achieve them. In that sense, the subject matter was irrelevant. Mr. Welsh taught us how to live.
Of course, the private lives of teachers are not necessarily obvious to their students, and I was certainly no less self-absorbed than your stereotypical teen-age knucklehead. I do remember the way that Mr. Welsh gushed about how amazing and heroic his wife was giving birth to their first child. But I couldn’t tell you what happened to him over the intervening 2 marriages and 20 years. All I can say is that of all the people I knew growing up, he was probably the last one I would expect to find on the tracks. That is what I find so heartbreaking.
To say that Mr. Welsh was a great teacher is an understatement. He was a legend who made the Robin Williams character in “Dead Poets Society” look like a clock puncher.
When I was a student 20 years ago, Mr. Welsh taught a class on politics and law, called IPLE (Introduction to Political and Legal Experience). This is not a natural point of interest for teenage boys and girls. So the way in which Mr. Welsh sucked kids into his classes is something we could all learn from, even if few have the talent and passion to emulate the man.
The class was not required for anything, be it the district, the state, or any of the colleges to which students aspired. But it was a popular class, taken by everyone from the Ivy League aspirants to the kids on parole.
First, consider what he didn’t do. He didn’t sex his classes up. It was what it was, and there was no bait-and-switch or fancy dressing. He wasn’t a pushover; his classes appealed because they were involved, not breezy. He didn’t dumb it down. You had to work hard, write well, and get out and do all sorts of non-traditional activities. He didn’t hammer it in. There was no fear, or guilt, or condensation. The material was there to be learned by whoever was interested. Instead, Mr. Welsh motivated his students to learn.
I don’t know if I can really explain why he was such a great teacher. Certainly he knew his subject matter, and explained it passionately. His IPLE class requirements made it personal, though grading contracts, hands-on exercises and non-traditional requirements. I still remember a classmate of ours getting class credit for explaining his day in court where he beat a driving-with-suspended-license charge. Mr. Welsh used these features to connect what would normally be an austere, esoteric body of knowledge to our every day lives.
But most importantly, he gave the encouragement necessary to convince us kids that we could use this knowledge, and the applications of it, to make our way in the world. It isn’t that he showed us how to be lawyers or pundits; very few of us did (although those few have generally done quite well). The lesson was more fundamental than that. He inspired us how to combine our knowledge and our passions to set goals and achieve them. In that sense, the subject matter was irrelevant. Mr. Welsh taught us how to live.
Of course, the private lives of teachers are not necessarily obvious to their students, and I was certainly no less self-absorbed than your stereotypical teen-age knucklehead. I do remember the way that Mr. Welsh gushed about how amazing and heroic his wife was giving birth to their first child. But I couldn’t tell you what happened to him over the intervening 2 marriages and 20 years. All I can say is that of all the people I knew growing up, he was probably the last one I would expect to find on the tracks. That is what I find so heartbreaking.