But those of you looking for subannual periodicity may be disappointed. I'm trying to finish a paper and progress has been slow. Do any of you parents or other obligatory multitaskers have useful strategies for keeping track of manuscript progress when available time is split up into 10-30 minute segments? I'm finding that by the time I figure out where I was up to the last time I was writing, the boy has thrown up on the carpet and the girl's run naked into the front yard, and writing time is over. I'm suffering from too much punctuation, and not enough equilibrium. Any tips?
p.s. The boy only sleeps 10 to 5:30, so getting up early only gives a small window.
I'm a geochemist. My main interest is in-situ mass spectrometry, but I have a soft spot in my heart for thermodynamics, poetry, drillers, trees, bicycles, and cosmochemistry.
Friday, July 30, 2010
Thursday, July 15, 2010
Is it wrong...
To cobble together a 40 page manuscript that doesn't quite make sense, submit it to a journal under a false name and address, and recommend 3 people you really don't like to be the reviewers?
Saturday, July 10, 2010
A different take on the PepSciBlog scandal
Much has been said of the PepSciBlog scandal, both by PepSciblings, the MSM, and the rest of the internet. I won’t repeat or comment on any of those opinions. Instead, I will try to prove that corporate shilling can be useful to basic science education, by using the Pepsi logo to explain the Rossiter-McLaughlin effect. Steinn should be ashamed that he didn’t beat me to this.
Imagine that in the days before spin, the Pepsi logo was designed by physicists, and thus was a featureless, luminous white sphere.

Figure 1. The pre-spin Pepsi logo.
This is fine for the Precambrian, but here in the 21st century, spin is very important. So we will spin the logo.

Figure 2. The spinning logo.
Spinning the sphere means that the side spinning away from us will be red shifted by the Doppler effect, while the side spinning toward us will be blue shifted. This is well illustrated by the modern logo.
This is fine, as long as the Pepsi logo spins alone, in the vastness of space. But interactions with other logos are important. Consider, for example, a transit, or partial eclipse, of the Pepsi logo by the Diabetes Australia logo.

Figure 3. Beginning of a diabetes transit of Pepsi.
If the direction of the transiting logo is the same as the direction of spin from the pepsi logo, then the light from the blue-shifted portion of the logo is blocked first. This makes the average observed Pepsi light somewhat redder. Later, as the Diabetes Australia logo moves to block the red-shifted part of Pepsi, the average light becomes bluer.

Figure 4. Late stage of a Diabetes Australia transit of Pepsi.
Like our hypothetical pre-spin Pepsi logo, stars are (approximately) luminous white spheres. So if they are rotating, the same effect can be observed when planets transit in front of them. More details of this effect can be found at the systemic blog. Thus education triumphs (until the evil lawyers shut me down).
Update:
A former Frink Tanker tells his story.
Imagine that in the days before spin, the Pepsi logo was designed by physicists, and thus was a featureless, luminous white sphere.

Figure 1. The pre-spin Pepsi logo.
This is fine for the Precambrian, but here in the 21st century, spin is very important. So we will spin the logo.

Figure 2. The spinning logo.
Spinning the sphere means that the side spinning away from us will be red shifted by the Doppler effect, while the side spinning toward us will be blue shifted. This is well illustrated by the modern logo.
This is fine, as long as the Pepsi logo spins alone, in the vastness of space. But interactions with other logos are important. Consider, for example, a transit, or partial eclipse, of the Pepsi logo by the Diabetes Australia logo.

Figure 3. Beginning of a diabetes transit of Pepsi.
If the direction of the transiting logo is the same as the direction of spin from the pepsi logo, then the light from the blue-shifted portion of the logo is blocked first. This makes the average observed Pepsi light somewhat redder. Later, as the Diabetes Australia logo moves to block the red-shifted part of Pepsi, the average light becomes bluer.

Figure 4. Late stage of a Diabetes Australia transit of Pepsi.
Like our hypothetical pre-spin Pepsi logo, stars are (approximately) luminous white spheres. So if they are rotating, the same effect can be observed when planets transit in front of them. More details of this effect can be found at the systemic blog. Thus education triumphs (until the evil lawyers shut me down).
Update:
A former Frink Tanker tells his story.
Thursday, July 08, 2010
Goldschmidt paleoblogging- the schmooze
Of course, one of the attractions of conferences, aside from the hot science and the big deals and the free* beer, is meeting the people whose papers I read, and catching up with colleagues and friends. And the conference did not disappoint in this regard either. I met a wide variety of ion probers, most of whom were friendly and smart. And I was pleasantly surprised at the number of people who I hadn’t seen in over a decade, who wandered up to me at some point and said g’day**. I saw people from Brown, people from field camp, people from grad school, and people from my technician days back at RSES. And many of the people, especially those who I hadn’t seen in ages, were doing awesome science. And if that wasn’t enough, I had the smallest possible geoblogger meetup (n=2) with petrological skepchick Evelyn, who is as enthusiastic and friendly in real life as she is on the internets.
So overall, it was a surprisingly fine meeting, not withstanding any overexertion on the part of my liver or my brain. They should both detox on the plane, which would be good since I miss my kids and wanna have a hangover-free play when I get home.
* excluding the conference fees, of course.
** or howdy or hello or whatever it is that they say in east Tennessee
So overall, it was a surprisingly fine meeting, not withstanding any overexertion on the part of my liver or my brain. They should both detox on the plane, which would be good since I miss my kids and wanna have a hangover-free play when I get home.
* excluding the conference fees, of course.
** or howdy or hello or whatever it is that they say in east Tennessee
Tuesday, July 06, 2010
2010 minimum arctic sea ice betting pool

Update 2!!
I think this is it, unless Eli and GFW and crandles wish ti reguess for reasons stated in the comments.
Update! The guesses so far are posted below. Contest will close tomorrow night once I get the kids to bed and clean up- call it 30ish hours from now. We're all a bunch of doom-glooming alarmists so far!

original post
The 2010 Arctic sea ice extent minimum prediction pool is now open.
Guesses are to be in the form of extent and sigma (a mathematical measure of uncertainty), in thousands of km2 You may use decimal places if you insist.
Your guess will define a Gaussian curve.
The function with the highest value for x=minimum daily measured ice extent (from IARC-JAXA) wins.
See the 2009 announcement, opening, and final curve for details.
I apologize for not posting on the solstice, but I was in transit from a conference in America.
This contest will close much sooner than last year's. Guesses must be submitted by the time the Earth reaches aphelion in its orbit, which the internet tells me is 11 am on July 6 (presumably UTC). Trash talking, dissembling, and boasting in the comments section is encouraged.
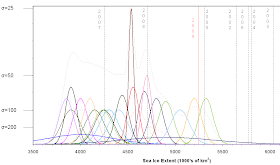
Figure 1. Last year's contestant curves and final 2009 minimum sea ice extent (red line).
As with last year, the winner gets to pick a topic for a silly blog post.
Sunday, July 04, 2010
More on the asbestos dust-up
Silver Fox has an excellent summary of serpentine group minerals, serpentinite rock, and the various asbestiform minerals. An even more interesting discussion has taken place on the pages of Elements magazine, the joint membership / review journal of a number of mineralogical and geochemical societies.
In June 2009, Micky Gunter wrote a scathing article on how mineralogists were being left out of the asbestos debate. This was complimented by an outreach article by Thomas Feininger on the use of Mg3Si2O5(OH)4 as the launching pad for all sorts of cool interdisciplinary and instructional mineralogy.
Four months later, Gregory Meeker of the USGS chimed in on the dark side of mineralogists in the asbestos debate, and Micky Gunter replied. The following part of Meeker’s article is worth quoting:
In short, the mining company mineralogists and lawyers knew perfectly well that their waste was just as deadly as ever, but they used a name change as a loophole to avoid culpability.
I originally came across this story with the intention of using it of an example of why nomenclature and other definition committees should not be too high-minded and ivory tower- in this case, the amphibole reclassification let the bad guys get away with murder. And this brings us to the comment section of the California bill:
Putting the idealized serpentine group formula in a sentence all by itself is extremely nebulous, but one way of reading this is that they are trying to define asbestos as Mg3(Si2O5)(OH)4. This would, of course, exclude the dangerous asbestiform amphiboles (e.g. Na2(Fe,Mg)5Si8O22(OH)2, or crocidolite) from the definition. This strikes me as asking for trouble.
Further reading:
Ann G. Wylie and Jennifer R. Verkouteren; Amphibole asbestos from Libby, Montana: Aspects of nomenclature; American Mineralogist; October 2000; v. 85; no. 10; p. 1540-1542
The Composition and Morphology of Amphiboles from the Rainy Creek Complex, Near Libby, Montana; G.P. Meeker, A.M. Bern, I.K. Brownfield, H.A. Lowers, S.J. Sutley, T.M. Hoefen and J.S. Vance; American Mineralogist; November-December 2003; v. 88; no. 11-12; p. 1955-1969
Mickey E. Gunter, M. Darby Dyar, Brendan Twamley, Franklin F. Foit, Jr. and Scott Cornelius; Composition, Fe3+/{sum}Fe, and crystal structure of non-asbestiform and asbestiform amphiboles from Libby, Montana, U.S.A.; American Mineralogist; November-December 2003; v. 88; no. 11-12; p. 1970-1978.
Mickey E. Gunter, Karen E. Harris, Kristin L. Bunker, Rebecca K. Wyss and Richard J. Lee; Amphiboles between the sheets: observations of interesting morphologies by TEM and FESEM; European Journal of Mineralogy; December 2008; v. 20; no. 6; p. 1035-1041; DOI: 10.1127/0935-1221/2008/0020-1872
Mickey E. Gunter, and Matthew S. Sanchez; Amphibole forensics: Using the composition of amphiboles to determine their source, the Libby, Montana, example; American Mineralogist; May-June 2009; v. 94; no. 5-6; p. 837-840; DOI: 10.2138/am.2009.3224
In June 2009, Micky Gunter wrote a scathing article on how mineralogists were being left out of the asbestos debate. This was complimented by an outreach article by Thomas Feininger on the use of Mg3Si2O5(OH)4 as the launching pad for all sorts of cool interdisciplinary and instructional mineralogy.
Four months later, Gregory Meeker of the USGS chimed in on the dark side of mineralogists in the asbestos debate, and Micky Gunter replied. The following part of Meeker’s article is worth quoting:
Gunter takes exception to a recent legal definition of asbestos; but there is more to that story. For over 70 years, the fibrous amphibole that is a major—not trace—constituent in the Vermiculite Mountain vermiculite deposit near Libby, Montana, was called tremolite, sodium-rich tremolite, or sodic tremolite by everyone including the mineralogists and geologists who studied the deposit. During the 1970s, the names of the regulated asbestos minerals, including tremolite asbestos, were entered into the U.S. Code of Federal Regulations. As recent court proceedings have revealed, company geologists, owners, and operators of the vermiculite mine near Libby understood that the asbestiform amphiboles in the mine fell under those regulations. In 1978 and 1997, committees of the International Mineralogical Association published new recommendations for amphibole nomenclature. Based on this new system of nomenclature, most of the amphibole minerals at the Libby mine were reclassified as winchite. When public and regulatory attention returned to Libby in 1999, mineralogists working on behalf of the company that owned the mine used the changes in nomenclature to claim that the majority of the Libby amphibole had been mistakenly identified as tremolite and therefore was not regulated. A federal judge sided with the defense and, based on a 2003 USGS study of the minerals, ruled that only 6 percent of the Libby asbestos was regulated.
In short, the mining company mineralogists and lawyers knew perfectly well that their waste was just as deadly as ever, but they used a name change as a loophole to avoid culpability.
I originally came across this story with the intention of using it of an example of why nomenclature and other definition committees should not be too high-minded and ivory tower- in this case, the amphibole reclassification let the bad guys get away with murder. And this brings us to the comment section of the California bill:
Chrysotile serpentine, also known as white asbestos, is the most common form of asbestos. Mg3(Si2O5)(OH)4.
Putting the idealized serpentine group formula in a sentence all by itself is extremely nebulous, but one way of reading this is that they are trying to define asbestos as Mg3(Si2O5)(OH)4. This would, of course, exclude the dangerous asbestiform amphiboles (e.g. Na2(Fe,Mg)5Si8O22(OH)2, or crocidolite) from the definition. This strikes me as asking for trouble.
Further reading:
Ann G. Wylie and Jennifer R. Verkouteren; Amphibole asbestos from Libby, Montana: Aspects of nomenclature; American Mineralogist; October 2000; v. 85; no. 10; p. 1540-1542
The Composition and Morphology of Amphiboles from the Rainy Creek Complex, Near Libby, Montana; G.P. Meeker, A.M. Bern, I.K. Brownfield, H.A. Lowers, S.J. Sutley, T.M. Hoefen and J.S. Vance; American Mineralogist; November-December 2003; v. 88; no. 11-12; p. 1955-1969
Mickey E. Gunter, M. Darby Dyar, Brendan Twamley, Franklin F. Foit, Jr. and Scott Cornelius; Composition, Fe3+/{sum}Fe, and crystal structure of non-asbestiform and asbestiform amphiboles from Libby, Montana, U.S.A.; American Mineralogist; November-December 2003; v. 88; no. 11-12; p. 1970-1978.
Mickey E. Gunter, Karen E. Harris, Kristin L. Bunker, Rebecca K. Wyss and Richard J. Lee; Amphiboles between the sheets: observations of interesting morphologies by TEM and FESEM; European Journal of Mineralogy; December 2008; v. 20; no. 6; p. 1035-1041; DOI: 10.1127/0935-1221/2008/0020-1872
Mickey E. Gunter, and Matthew S. Sanchez; Amphibole forensics: Using the composition of amphiboles to determine their source, the Libby, Montana, example; American Mineralogist; May-June 2009; v. 94; no. 5-6; p. 837-840; DOI: 10.2138/am.2009.3224
Saturday, July 03, 2010
In defense of serpentine
There is a move by asbestos activists and litigators in California to remove the status of state rock from serpentine. The language of the bill is heavy on the health effects of asbestos, particularly amphibole asbestos (which has no relation to serpentine at all). It makes some completely inaccurate statements, such as the allegation that all serpentines contain asbestos. It downplays, or completely ignores several of the key benefits of serpentine. So, three simple reasons why serpentine is great:
1. Serpentine sequesters CO2. Under atmospheric conditions, serpentine reacts with carbon dioxide in the air to form magnesite + quartz + water, via numerous intermediate hydrous magnesium carbonates. Mineral carbonation of serpentine is one of the most promising methods of removing CO2 from the atmosphere.
2. Serpentine prevents earthquakes. In central California, the portion of the San Andreas fault that cuts through serpentine bedrock does not suffer from catastrophic earthquakes. Instead it slowly and peacefully creeps. The 1989 Loma Prieta earthquake, which killed 63 people, could have been as big as the 1906 San Francisco Earthquake, which killed more than 3000 people, were it not for the presence of serpentine (and alteration products) in the fault to the south of the rupture area.
3. Serpentine may be responsible for life on Earth. The formation of serpentine from the reaction between water and the Earth’s mantle releases hydrogen gas, which combines with CO2 to form methane and organic molecules. This hydrogen is a food source for methanogens, one of the Earth’s most primitive and ancient life forms, and the unusual chemistry associated with serpentine formation could well have provided the building blocks and energy source for the birth of all of life on earth, including the California state legislature.
In short:
There are 20 forms of serpentine, only one of which is an asbestos mineral.
The very dangerous amphibole asbestos minerals specifically mentioned in the bill are completely unrelated to serpentine.
Safety disclaimer: The inhalation of ANY rock dust is harmful to the lungs. Asbestos dust is particularly dangerous. Do not breathe rock dust.
1. Serpentine sequesters CO2. Under atmospheric conditions, serpentine reacts with carbon dioxide in the air to form magnesite + quartz + water, via numerous intermediate hydrous magnesium carbonates. Mineral carbonation of serpentine is one of the most promising methods of removing CO2 from the atmosphere.
2. Serpentine prevents earthquakes. In central California, the portion of the San Andreas fault that cuts through serpentine bedrock does not suffer from catastrophic earthquakes. Instead it slowly and peacefully creeps. The 1989 Loma Prieta earthquake, which killed 63 people, could have been as big as the 1906 San Francisco Earthquake, which killed more than 3000 people, were it not for the presence of serpentine (and alteration products) in the fault to the south of the rupture area.
3. Serpentine may be responsible for life on Earth. The formation of serpentine from the reaction between water and the Earth’s mantle releases hydrogen gas, which combines with CO2 to form methane and organic molecules. This hydrogen is a food source for methanogens, one of the Earth’s most primitive and ancient life forms, and the unusual chemistry associated with serpentine formation could well have provided the building blocks and energy source for the birth of all of life on earth, including the California state legislature.
In short:
There are 20 forms of serpentine, only one of which is an asbestos mineral.
The very dangerous amphibole asbestos minerals specifically mentioned in the bill are completely unrelated to serpentine.
Safety disclaimer: The inhalation of ANY rock dust is harmful to the lungs. Asbestos dust is particularly dangerous. Do not breathe rock dust.