Evidently there's a
conference in Maryland this week that will be discussing planetary definition. So I figured I'd throw a little something together about when planets grow up.
Back in 2006, the IAU kicked Pluto out of the planet club, as I
cynically predicted they would. Their new planetary definition is a bit odd, though. For one thing, it is heliocentric- planets must orbit the sun, and not any of the other estimated 99,999,999,999 stars in this galaxy (or any of the countless stars in other galaxies) in order to fit the definition. In addition, a planet has to be round (no squares in this club, ya hear Poindexter?), and it has to have cleared its orbit of any rivals.
Not long after, Hal Levinson parameterized this orbit clearing with a handwavy yet quantitative derivation which can be found
on his website. He assumes that a planet can clear its orbit by either accreting or ejecting everything around it, and shows the necessary equations before plotting them against a number of the solar systems larger objects. There’s even a nice graph that shows which bodies make the planetary cut, and which are nonplanetary combatants, or whatever these second class orbiters are called these days:
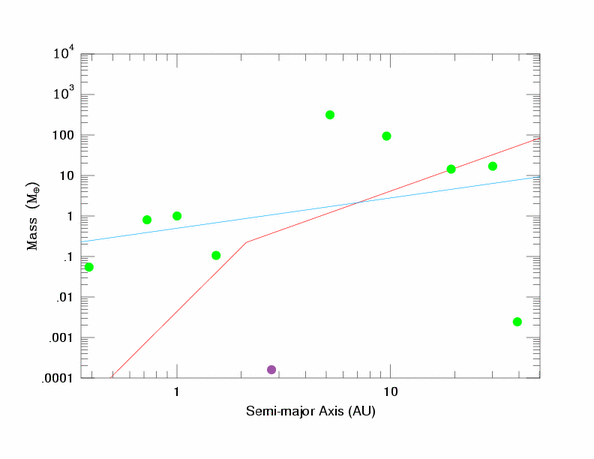
As y’all know, I’m an ex-geochronologist. So to me when is just as interesting as what. I noticed that all of these equations, written to solve for planetary mass, could be rearranged to solve for t. Instead of determining if something is a planet, we can instead find out when it achieved planetary status.
As it turns out, the accretion formulas, which are more important than ejection for the inner solar system, are strongly dependent on the orbital inclination of the stuff which they are trying to pick up. I don’t know what value for i Dr. Levinson used, but I suspect it was rather large, as small i values lump Earth in with the gas giants for the purposes of gravitational focusing in the accretion equation. So below is a chart showing how long after solar system formation each planet will need to graduate from dwarf planet to true planetary status.
Note that the formula, because of its arm wavyness, assumes constant orbital radius, constant mass, etc etc etc (follow the link to Hal’s page for details).
Inclination is in degrees, time is myr since formation.
| inclination | 5 | 10 | 15 | 20 | 25 |
| mercury | 11.0 | 22.9 | 34.2 | 45.2 | 55.8 |
| venus | 1.6 | 12.7 | 42.1 | 68.9 | 85.1 |
| earth | 2.5 | 19.9 | 65.8 | 151.9 | 227.2 |
| mars | 125.5 | 992.6 | 2139.7 | 2827.5 | 3493.8 |
| jupiter | 0.04 | 0.12 | 0.12 | 0.12 | 0.12 |
| saturn | 0.82 | 3.27 | 3.27 | 3.27 | 3.27 |
| uranus | 70.2 | 397.6 | 397.6 | 397.6 | 397.6 |
| neptune | 189.1 | 563.9 | 563.9 | 563.9 | 563.9 |
| ceres | 296368.3 | 590481 | 880099.812 | 1163021 | 1437090 |
| pluto | 61727948 | 4.88E+08 | 1616525312 | 2.16E+09 | 2.67E+09 |
| eris | 1.74E+08 | 1.37E+09 | 4545118377 | 1.05E+10 | 1.53E+10 |
| make make | 4.33E+08 | 3.42E+09 | 5993005070 | 7.92E+09 | 9.79E+09 |
So, what do we see?
Firstly, Jupiter and Saturn don’t remain dwarf planets for very long. This is unsurprising, as they are in fact giants. Conversely, the time for Ceres and the Kupier belt objects of unusual size (Plutonoids? Plutoids? I just wish these objects were massive enough to prevent their names from evaporating away) to become planets is longer than the expected lifetime of the Sun. The ice giants and terrestrial planets are all intermediate.
Of these, however, the most interesting one is Mars. Mars spends a considerable fraction of its lifetime (between 3% and 77%, depending on inclination) as a pre-planet. And even for the fast, low angle accretion, the time is considerably longer than the 30Ma Hf-W age that comes from real rocks. So if the red planet did harbor life during its first billion years before drying out and freezing, it would have done so as a non-planetary object, according to the IAU. Indeed, waiting for a young, or even middle aged planet to fling the last interloping object out of its path to gain a title is a decidedly odd proposition. Depending on where you start the planetary clock ticking, I may have even worked on terrestrial zircons from Earth’s wild pre-planetary youth.
The start time question is important. From a geochemical point of view, a planet is 'born' when the core and mantle stop exchanging mass. The orbit doesn't really matter. But planetary orbits are not always constant. So for the purposes of planetary orbit clearing, it is probably the time since reaching their current orbital configuration, and not their original orbit, that matters. Which for our system would make T
0 3850 Ma instead of 4567Ma, if we accept the Nice model. Applying the current IAU planet definition to migrating planets is so much fun that it will require a post of its own, which I’ll try to get up soon.















